A 21-year-old woman developed lethal toxic blood after taking a popular weight-loss drug for just three weeks.
The unnamed patient, who had been injecting a medication from the same class as Ozempic, was admitted to the hospital after two days of abdominal pain, vomiting and diarrhea.
Blood tests revealed he was suffering from a life-threatening condition called ketoacidosis, which occurs when fat-melting proteins called ketones build up in the blood.
In small, short-term doses, ketones are relatively harmless and can accelerate weight loss. But in large quantities, they cause the blood to become too acidic, causing catastrophic heart problems.
The patient, whose case was documented in the European Journal of Case Reports in Internal MedicineHe was found to have 26 times the normal level of ketones in his blood.
An unnamed patient in Kuwait suffered ketoacidosis after taking tirzepatide, the active ingredient in the successful weight loss drugs Mounjaro and Zepbound.
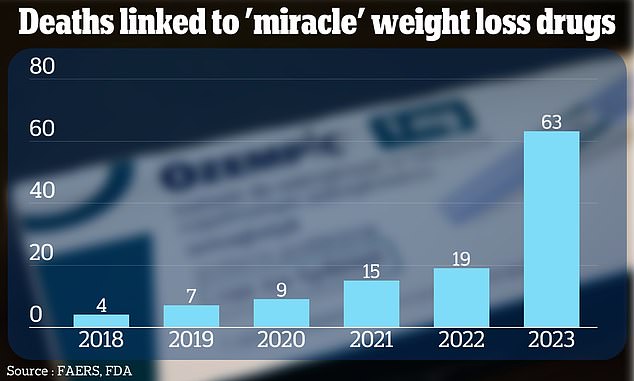
The cases have been recorded in an FDA tracking system used to track the safety of drugs used in the US, called FAERS. They are shown above in a graph.
Three weeks earlier, he began taking tirzepatide, the active ingredient in the successful weight-loss drugs Mounjaro and Zepbound, once a week. She had lost about 11 pounds.
The complication is one of a long list of side effects linked to GLP-1 agonists such as Ozempic and Mounjaro, which have also been linked to more than 100 deaths in the United States.
Normally, the body breaks down glucose, or sugar, from food and converts it into energy.
But if the body doesn’t get enough, it breaks down fat stores as backup. This chemical reaction produces ketones as a byproduct.
This might be more likely in patients taking GLP-1 agonists like Ozempic or Mounjaro because the drugs suppress appetite, meaning patients don’t get much energy from food.
Ketoacidosis is typically seen in diabetic patients as their bodies cannot produce enough insulin, which helps convert food into energy, letting fat break down.
Symptoms include excessive thirst, frequent urination, vomiting, stomach pain, weakness, difficulty breathing, confusion, and fruity breath; This is because ketoacidosis produces high levels of the chemical acetone, which has a characteristic fruity odor.
If left untreated, ketoacidosis can lead to low blood sugar, brain inflammation, and death.
At the hospital, the patient, from Kuwait, suffered severe breathing problems, elevated heart rate and high blood pressure.
He was given intravenous fluids to restore his sodium and potassium levels and stop the vomiting and diarrhea.
Four weeks later, his symptoms disappeared, although he had gained two pounds. The patient was advised to stop taking tirzepatide.
Ketoacidosis joins a long list of complications related to blockbuster weight-loss medications.
Patients previously told DailyMail.com they suffered organ failure, stomach paralysis and suicidal thoughts.
They include a mother who suffered kidney failure on her daughter’s birthday and a man whose sister begged him not to try to kill himself a third time after Ozempic “ruined” her life.
Ashley Keenan, 37, suffered from diabetic ketoacidosis (DKA) in 2021 after several months taking Ozempic.
She told DailyMail.com that her potassium level dropped so low that her heart stopped.
He then had to spend 10 days in the ICU with DKA, gallstones and pancreatitis (inflammation of the pancreas).


Ashley Keenan (left) suffered diabetic ketoacidosis after taking Ozempic. She told DailyMail.com that her potassium level dropped so low that her heart stopped. Brea Hand (right) said she was diagnosed with gastroparesis after taking the medication.
‘For the first 48 hours, they weren’t sure if I would make it. “I can’t tell you how strange it is to have doctors visit you from the emergency room to see if you survived,” Mrs. Keenan said.
“My body had become acidic and my organs were shutting down.”
Additionally, a DailyMail.com analysis of FDA data revealed that more than 100 deaths have been linked to these weight loss drugs.
In total, the FDA system has recorded 117 deaths among people taking blockbuster weight-loss drugs since 2018.
Of those, 81 were related to patients using semaglutide (the active ingredient in Ozempic and Wegovy), while 36 were related to patients using tirzepatide.
Symptoms recorded ranged from seizures to blockages in the intestines and pancreatic cancer.
Experts told DailyMail.com they were surprised that the number of deaths linked to weight-loss drugs was “not higher.”

