Health authorities have approved a new blood test to detect colon cancer.
The FDA announced Monday that it has approved Shield, a blood test from California-based Guardant Health.
While the test is not intended to replace colonoscopies (the gold standard for screening), doctors believe it could help combat the rise in colorectal cancer cases across the United States, particularly among young people.
And the tool, which currently sells for nearly $900, will likely be covered by most insurance plans, though it’s unclear when that coverage will begin.
Guardant’s blood test, which currently sells for $895, is expected to be covered by most insurance plans following FDA approval.
Dr. Sapna Syngal, director of strategic planning for cancer prevention and early detection at Dana-Farber Cancer Center in Boston, said: NBC News‘The biggest problem with colon cancer at the moment is that a significant portion of the population does not undergo screening.’
“If this test increases the number of people being tested, it will have a huge impact.”
Guardant recommends that Shield be administered every three years, starting at age 45 (the same age at which colonoscopies are recommended to begin).
If Shield detects cancer DNA, a colonoscopy will still be needed to locate tumors.
Dr. Robert Smith, senior vice president of early cancer detection sciences at the American Cancer Society, told NBC News: “People need to understand that a positive Shield test requires a colonoscopy to confirm that you have an advanced lesion or colorectal cancer, or that the results were false.”
‘A test like this is not complete if it’s positive and you haven’t had a colonoscopy.’
The approval comes after a March study found Shield was 83 percent effective at detecting colorectal cancer, though it is best suited to detecting late-stage disease.
The study found that the test, which detects DNA released by cancerous tumors into the bloodstream, was only 13 percent effective at detecting the disease at an early stage.
According to the NCI, about one in four colorectal cancers are detected at stages three or four, and less than 20 percent of patients with stage four disease will survive after five years.
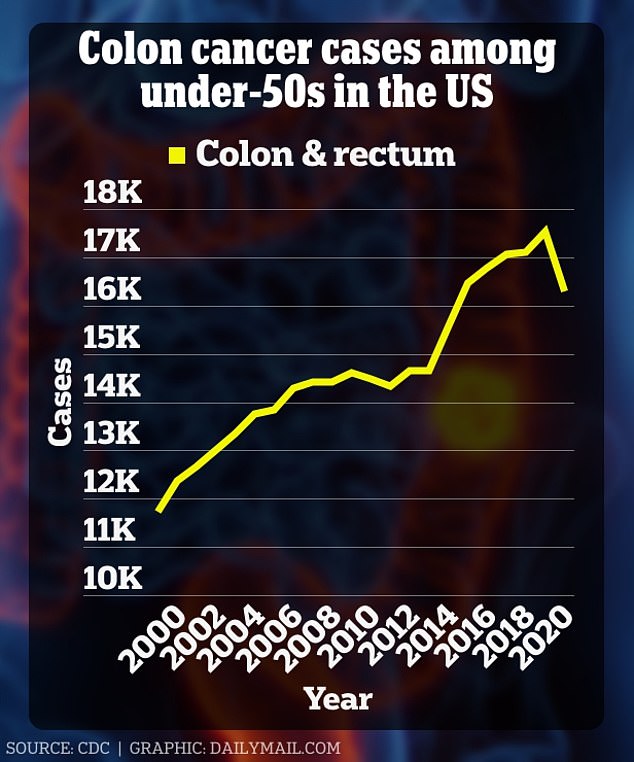
The American Cancer Society estimates that more than 53,000 people will die from colon cancer this year, and that number has risen especially among Americans under age 50.
Scientists are still trying to figure out what exactly is behind this increase, but recent research suggests that diets high in red meat, processed foods and sugar could be to blame.
And researchers at the University of Florida are recruiting young patients to determine whether energy drinks containing taurine might also cause colorectal cancer.
Dr. Arvind Dasari, an associate professor in the department of gastrointestinal and medical oncology at the University of Texas MD Anderson Cancer Center, told NBC News that the approval was a “positive development,” though he cautioned that the exact impact remains unclear.
We will have to wait and see what the impact will be in terms of improving detection and reducing the incidence of mortality.”