Elon Musk’s Neuralink has been given the green light to implant its brain chip in a second patient after fixing issues that arose during the first human trial.
The U.S. Food and Drug Administration approved the next person on Monday, signing off on the company’s planned upgrades that included embedding some of the device’s ultra-thin wires deeper into the brain.
Neuralink revealed this month that some of the 64 threads became detached from the first patient’s brain, causing the chip to malfunction, nearly ending the trial that began in January.
A Reuters report cited “five people familiar with the matter” who had stated that this problem had been “known for years” thanks to animal testing.
Elon Musk’s Neuralink has been given the green light to implant its brain chip in a second patient after fixing issues that arose during the first human trial.
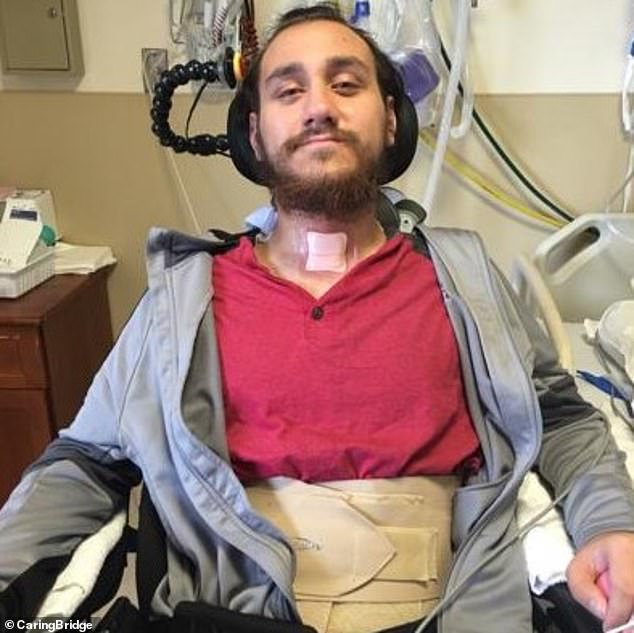
In January, Neuralink implanted a brain chip in its first patient, Noland Arbaugh, who is paralyzed from the shoulders down due to a 2016 diving accident.
Nolan Arbaugh was the first to receive the brain chip after a life-changing car accident while working as a camp counselor in 2016, which left him with “absolutely no feeling” from the shoulders down.
Neuralink shared a progress update on Arbaugh on May 8, announcing that it had been more than 100 days since he was implanted with the device.
However, the company also revealed that some of the threads connected to the chip had retracted weeks after the surgery, resulting in a decrease in the number of effective nodes.
A report of the Wall Street Journal He claimed the problem arose from the initial surgery when air became trapped inside Arbaugh’s skull, which is known as pneumocephalus and can cause seizures, brain abscesses and death if left untreated.
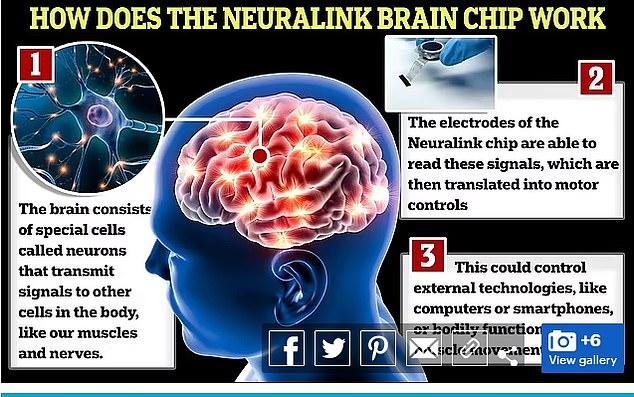
The US Food and Drug Administration (FDA) approved the next person on Monday, signing off on the company’s planned upgrades that included embedding some of the device’s ultra-thin wires deeper into the brain.
The Journal also reported that the FDA is allowing Neuralink to move forward with a second patient, which comes days after the company opened applications.
The implant is about the size of a quarter and is equipped with electronic components and a battery.
The threads are inserted into the brain’s motor cortex, the region that generates signals to direct the body’s movement.
Arbaugh told The Journal that 15 percent of the threads had remained in his brain after the malfunction.
Neuralink was able to modify the algorithm to increase signal translations without having to remove the chip.
The company is testing its implant to give paralyzed patients the ability to use digital devices while thinking on their own, a prospect that could help people with spinal cord injuries.
The company is now targeting June for the implantation of the second patient and aims to have 10 people with the chip this year.

The implant is about the size of a quarter and is equipped with electronic components and a battery. The threads are inserted into the brain’s motor cortex, the region that generates signals to direct the body’s movement.
So far, the device has allowed Arbaugh to play video games, browse the Internet and move a computer cursor on his laptop by thinking alone, according to company blog posts and videos.
Neuralink also noted that shortly after surgery, Arbaugh broke the world record for the speed at which she can control a cursor with her thoughts alone.
Just a few weeks after surgery, Arbaugh was able to control her laptop using Link, which she did to play computer games with friends, browse the Internet, live stream, and use other applications on her MacBook.
“(The Link) has helped me reconnect with the world, my friends and my family,” he said.
“It has given me the ability to do things on my own again without needing my family at all hours of the day and night.”
Arbaugh spends up to eight hours a day contributing to research, but spends more than 10 hours a day on weekends on personal activities.
Neuralink said it recently used the device for a total of 69 hours in a single week: 35 hours of structured sessions and an additional 34 hours of personal use.
The company mentioned that some of the wires connected to the chip had retracted from the brain, but the Neuarlink team modified the recording algorithm to make it more sensitive to signals from the neural population.
This resulted in “better techniques for translating these signals into cursor movements and an improved user interface.”
Neuralink said it is now focusing on increasing cursor control performance to the same level as that of healthy people.
“In the future we intend to expand Link’s functionality into the physical world to enable control of robotic arms, wheelchairs and other technologies that can help increase the independence of people living with quadriplegia,” the company said.

