AstraZeneca’s historic decision to withdraw its Covid vaccine worldwide was hailed by victims of an extremely rare but fatal side effect, who said it “means no one else will suffer this terrible adverse reaction”.
The pharmaceutical titan’s vaccine, once heralded as a “triumph for British science”, has come under intense scrutiny in recent months for a very rare complication that causes blood clots and low blood platelet counts. It has been linked to 81 deaths in the UK, as well as hundreds of serious injuries.
The vaccine, developed with the University of Oxford, can no longer be used in the European Union after the company voluntarily withdrew its “marketing authorisation”, which comes into effect today.
Similar requests to withdraw the vaccine will be made in other countries that had previously approved it, including the UK. Around 50 million doses have been administered in Britain.
AstraZeneca’s withdrawal comes months after it admitted that its vaccine can cause the thrombosis reaction with thrombocytopenia syndrome (TTS).
One of those seeking compensation for injuries related to the AstraZeneca vaccine is computer engineer Jamie Scott, a father of two. He was left with a permanent brain injury following a blood clot and brain hemorrhage after receiving the vaccine in April 2021. The 47-year-old has not been able to work since.
One of those seeking compensation for injuries related to the AstraZeneca Covid vaccine is father-of-two and computer engineer Jamie Scott (right). His wife Kate (left) said she hoped AstraZeneca’s new filing was a sign that the legal case could be resolved soon.
His wife Kate said: “The fact that the AstraZeneca Covid vaccine is no longer being used in the UK or Europe, and soon the rest of the world, means that no one else will suffer this terrible adverse reaction.”
“They say it’s for commercial reasons, but maybe it’s because it can no longer be considered within acceptable safety parameters.”
Scott, 47, told Daily Telegraph: ‘This is good news, but I will always wish that, like they did in other countries, they had stopped him in the UK after just one case.
“More lives could have been saved and I wouldn’t be suffering like I am.”
Fifty-one families are currently taking legal action against the pharmaceutical titan, arguing that its “defective” vaccine was to blame for the injuries and deaths of their loved ones.
However, lawyers argue that we may never know the true number of people affected by the rare but devastating complication of TTS.
Sarah Moore, partner at law firm Leigh Day, told MailOnline: “The criteria for what constituted TTS were not actually published and were available to the clinical community until early March (2021).”
He added that as the complication was only detected when the vaccine began to be administered to younger people, cases in older people could have gone unnoticed and been confused with Covid-related problems or other health problems.
“We may never know if there were other injuries that could have been related to the vaccine before March 2021,” he said.
Moore added that the company had been “inundated” with people coming forward claiming they or a family member had been affected by the AstraZeneca vaccine, but some had to be turned away.

The AstraZeneca vaccine was the most widely used in the UK during the initial rollout of the vaccination programme, before it was linked to a risk of blood clots.
“Unfortunately, for various reasons, it is not possible for us to address all the cases that come before us,” he said.
He added that some potential victims had run out of time to seek compensation.
“For the claims we file, injured or bereaved people have three years from the date of their injury or death to file a claim, so unfortunately in many cases that limit has already been reached,” he said.
AstraZeneca denies that the decision to withdraw the vaccine is related to the court case, insisting that the vaccine is instead being withdrawn from the markets for commercial reasons.
The company said in court documents that the vaccine is reportedly no longer manufactured or supplied, having been replaced by updated vaccines that address newer variants.
A spokesperson said: “We are incredibly proud of the role Vaxzevria played in ending the global pandemic.
‘According to independent estimates, more than 6.5 million lives were saved in the first year of use alone and more than three billion doses were delivered worldwide.
‘Our efforts have been recognized by governments around the world and are widely considered a critical component in ending the global pandemic.
‘As multiple variants of Covid vaccines have since been developed, there is a surplus of updated vaccines available.
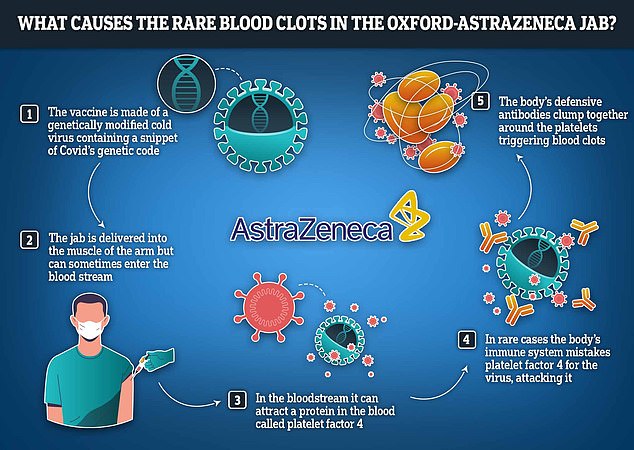
Researchers believe this rare side effect is because the modified cold virus hiding in the injection has an adverse effect on platelets in the blood, causing them to clot.
‘This has led to a decline in demand for Vaxzevria, which is no longer manufactured or supplied. For this reason, AstraZeneca has made the decision to begin the withdrawal of marketing authorizations for Vaxzevria in Europe.
“We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and a significant contribution to the Covid pandemic.”
TTS, or vaccine-induced immune thrombotic thrombocytopenia (VITT), is thought to be linked to at least 81 deaths in the UK.
However, not all of them are proven. And not all families seek legal action.
TTS is when a person suffers from blood clots along with a low platelet count. Platelets often help blood clot.
The complication, listed as a possible side effect of the vaccine, was previously called vaccine-induced immune thrombotic thrombocytopenia (VITT).
The complication is extremely rare, given the millions of doses distributed during the rollout. The risk is believed to be one in 50,000.
AstraZeneca’s admission in court papers earlier this year could lead to case-by-case payments.
Taxpayers will foot the bill for any potential deal because of a compensation deal AstraZeneca struck with the Government in the darkest days of Covid to get jabs produced as quickly as possible while the country was paralyzed by lockdowns.
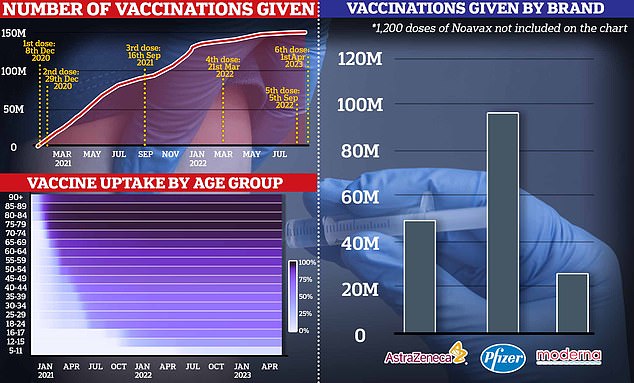
The graph shows the cumulative number of Covid jabs administered in the UK since the pandemic began, the percentage of each age group who have received a jab (bottom left) and the number of each brand of Covid vaccine administered.
Similar actions to those being taken by British families are understood to be taking place in other countries where the AstraZeneca vaccine has been rolled out, including Germany and Italy.
Health officials first identified cases of VITT linked to the AstraZeneca vaccine in Europe as early as March 2021, just over two months after the vaccine was first rolled out in the United Kingdom.
However, it was not until April of that year that the evidence became clear enough for the vaccine to begin to be restricted.
Frightened officials first restricted the vaccine to only people over 30. Then, in May 2021, they reduced it only to those over 40 years of age.
As the vaccine still worked against Covid, it was still considered worth giving to older Britons who were at higher risk of death or injury from falling ill with the virus.
The new Covid vaccine rollouts have minimized the use of the AstraZeneca vaccine and/or eliminated it entirely in favor of mRNA alternatives such as those made by rival pharmaceutical giants Pfizer and Moderna.

