Campaigners hit back today after health chiefs revealed NHS patients with Alzheimer’s would miss out on a “revolutionary” drug proven to combat the devastating disease.
Experts have long believed that donanemab could herald a new era in dementia treatment, after studies showed it slowed the memory-robbing disease in its early stages.
Today it was approved by the medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA).
However, in a blow to tens of thousands of Britons, Draft guidance from the NHS spending watchdog ruled that the benefits of the drug could not justify the cost of implementation.
It means that donanemab will only be available to those who can pay privately each year, unless they are part of a clinical trial.
Experts have long believed that donanemab could herald a new era in dementia treatment, after studies showed it slowed the memory-robbing disease in its early stages.
Alzheimer’s disease is the most common cause of dementia. The disease can cause anxiety, confusion and short-term memory loss.
It is also believed that health insurance policies are unlikely to cover the costs.
Campaigners and charities today called the decision “incredibly disappointing” and warned that “the UK is no longer a good place to launch new dementia treatments.”
It is estimated that around 70,000 adults in England would have been eligible to receive donanemab if it had been approved for use in the health service.
It is also the second time NICE has rejected a new Alzheimer’s treatment in a matter of months.
In August, it ruled that the benefits of lecanemab (hailed as “the beginning of the end” for dementia last year) “are too small.”
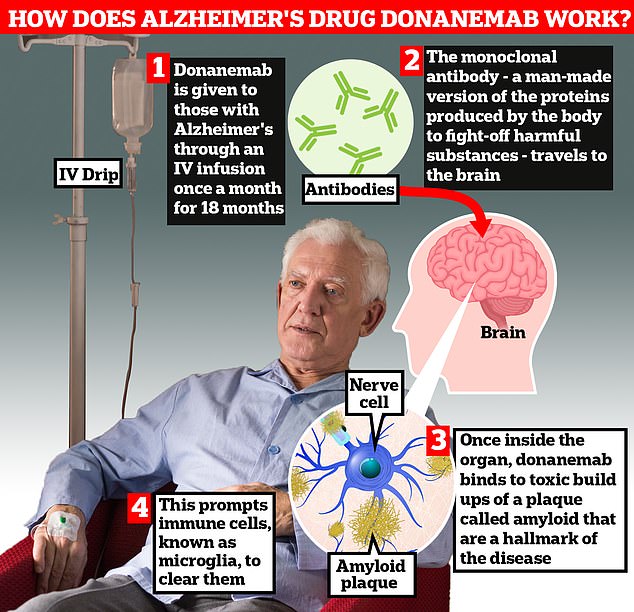
Donanemab has been shown to slow disease progression by up to 35 percent in trials by helping to eliminate the buildup of the harmful protein amyloid in the brains of people with early-stage Alzheimer’s.
Known as amyloid immunotherapy, these proteins are believed to interfere with messages sent between different parts of the brain, causing symptoms of memory loss and independence.
Patients received donanemab via a drip in the arm in the hospital every month for 18 months in clinical trials.
But experts said additional monitoring for side effects, including brain scans, would mean additional costs could skyrocket.
Currently, the only medicines available on the NHS for Alzheimer’s are to treat the symptoms.
Helen Knight, NICE’s director of medicines evaluation, said: “For NICE to approve a medicine for use in the NHS, it must provide additional benefits to patients and must also represent a good use of NHS and healthcare resources. taxpayers’ money.”
‘Our independent committee examined all the available evidence, including the benefits for carers.
‘This shows that donanemab could slow cognitive decline by 4 to 7 months, but it is not enough of a benefit to justify the additional cost to the NHS.
“The cost-effectiveness estimate for donanemab is 5 to 6 times higher than what NICE normally considers an acceptable use of NHS resources.
“I know it will be disappointing news, but this is an emerging field of medicine and other treatments are being developed.”
NICE also said it had identified a further 27 medicines that it “expects to be asked to evaluate in the coming years”.
The drug has been shown to slow disease progression by up to 35 percent in trials by helping to eliminate the buildup of the harmful protein amyloid in the brains of people with early-stage Alzheimer’s.
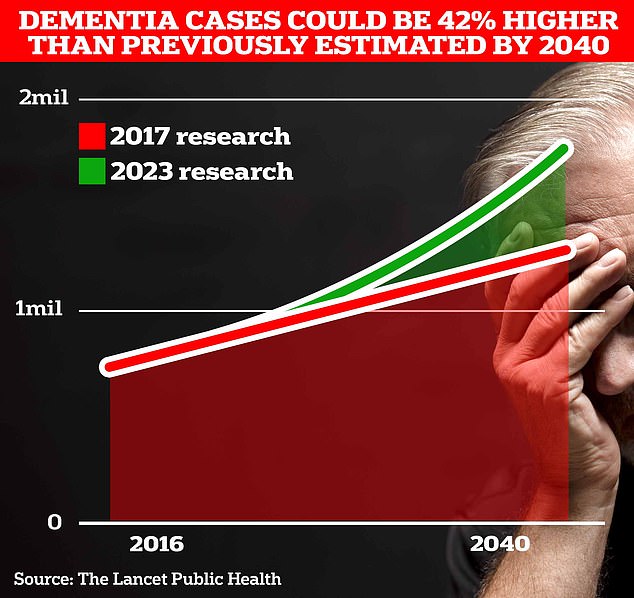
It is currently believed that around 900,000 Britons suffer from this memory-robbing disorder. But scientists at University College London estimate this figure will rise to 1.7 million within two decades as people live longer. It marks a 40 percent increase from the previous forecast in 2017.
However, today charities and campaigners criticized the “frustrating setback” and warned that “the UK is no longer a good place to launch new dementia treatments”.
Hilary Evans-Newton, chief executive of Alzheimer’s Research UK, said: “Today’s announcement marks another frustrating setback for people affected by Alzheimer’s disease.
‘We finally have two new treatments authorized in Britain for Alzheimer’s.
“But it is incredibly disappointing that NHS patients in England and Wales will not receive them.
‘While these drugs are not cures and carry risks of side effects, trials show that they are the first treatments that slow the decline in memory and thinking skills related to Alzheimer’s, rather than just relieving symptoms.
‘NICE’s recent interim decisions on lecanemab and donanemab highlight the uncertainty over their benefits compared to the significant costs of administering them in the NHS.
“However, dementia remains the leading cause of death in the UK and, if action is not taken, the aging population means more families will be affected, increasing NHS costs through admissions and emergency care”.
NICE also said it had identified a further 27 medicines that it “expects to be asked to evaluate in the coming years”.
He added: ‘NHS England has identified almost 30 more dementia treatments that could be available by 2030, giving the government and the NHS a crucial opportunity to transform the way dementia is treated, as promised. the Labor Party in its manifesto.
“But we have not yet heard from Health Secretary Wes Streeting on how he plans to break the impasse we face, where research is yielding new treatments but they remain out of reach for NHS patients.
“Today’s decision also risks signaling that the UK is no longer a good place to launch new dementia treatments.”
The UK is also home to only 7 per cent of the world’s dementia trials, he noted.
“How the government addresses these challenges will demonstrate whether it is serious about bringing innovation to the NHS and reducing the bureaucracy that limits people’s access to research and innovative medicines,” he said.
Meanwhile, Professor Fiona Carragher, director of policy and research at the Alzheimer’s Society, said: “The MHRA’s approval of donanemab marks another milestone on this journey, but is accompanied by a draft NICE decision not to recommend donanemab for use in the NHS.
“While this is discouraging, we respect the regulator’s decision.”
He added: ‘New treatments are an important catalyst for change, but they are just one piece of the puzzle.
‘As we prepare for the future, we must not lose sight of the one million people living with dementia in the UK today, a third of whom do not have a diagnosis.
‘We need to see significant government investment to achieve radical change so that all people with dementia in the UK can get an early and accurate diagnosis.
“Without this, people will not be able to access existing treatments and interventions to help manage their symptoms today or be prepared for tomorrow’s treatments that will curb the disease.”
It comes as it was revealed in April that around 5,000 Britons could have cheap blood tests to detect Alzheimer’s in an effort to revolutionize the NHS’s “shocking” diagnosis rates.
In two landmark trials, researchers from Oxford and University College London will use tests to detect proteins in the blood linked to the disease.
At the time, researchers said they hoped the “innovative” blood test, which costs around £100, could speed up the process, allowing patients to receive treatment sooner.
A recent analysis by the Alzheimer’s Society estimates that the total annual cost of the disease is £42 billion a year, with families hardest hit.
But population growth and aging means the charity estimates these costs – which include lost income from unpaid carers – will soar to £90bn over the next 15 years.
Around 944,000 people in the UK are thought to be living with dementia, while in the US the figure is around 7 million.
Alzheimer’s affects approximately six in 10 people with dementia.
It is believed to be caused by a buildup of amyloid and tau in the brain, which build up and build up of plaques and tangles that make it difficult for the brain to function properly.
Over time, the brain struggles to cope with this damage and symptoms of dementia develop.
Memory problems, thinking and reasoning difficulties, and language problems are common early symptoms of the condition, which then worsen over time.
Dementia is expected to skyrocket in the coming years, so a cheap screening tool is vital to meet the challenge.
Analysis by Alzheimer’s Research UK found that 74,261 people died from dementia in 2022, compared to 69,178 the previous year, making it the leading cause of death in the country.