A new analysis has revealed the dangers of buying private label medications from CVS.
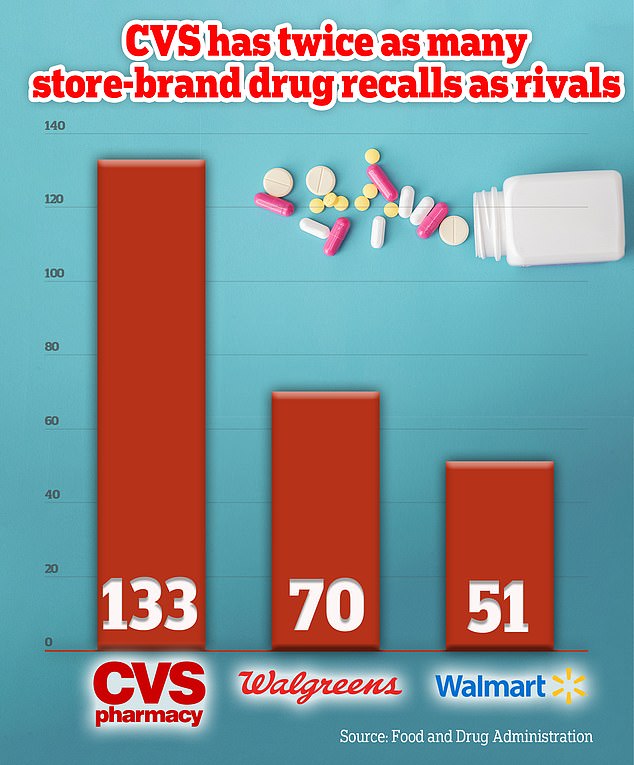
FDA data shows that America’s largest pharmacy chain has recalled 133 over-the-counter medications over the past decade, or about one a month.
That was more than twice as many as competitor Walgreens, which had 70 recalls during the same period, and three times as many as Walmart, which had 51 recalls.
Reasons for the CVS recalls included medications infested with bacteria, mold growing on factory fans, peeling paint, barefoot factory workers and pills containing incorrect dosages.
Private label eye drops were the CVS products most likely to be recalled over the past decade, followed by private label constipation medications (such as magnesium citrate tablets) and cold medications. and flu.
An analysis of recalls showed that over the past decade CVS has recorded 133 recalls, at least twice as many as its two closest rivals.

Pictured above is the factory run by Kilitch Healthcare India Limited, which made eye drops that were sold in stores like CVS, where they also carried the company’s label.
The recalled products were manufactured by companies based in China and India, as well as some in the United States, including Tennessee and Florida.
CVS has seen its recalls increase in recent years, recording fewer than ten per year between 2014 and 2018, but above this number for four of the six years since then.
So far this year, the chain has recorded 11 recalls, mainly for eye drops, cough medicines and medications to treat constipation.
Experts have long warned about generic drugs, stating that there are very few incentives for pharmacy chains to guarantee their quality.
This is due to a loophole in FDA rules that makes CVS not responsible for the quality of generics made by third-party factories, even when the products bear a red heart and the words “CVS Health.”
said Dr. Kevin Schulman, a medical expert at Stanford University. Bloomberg: “The best way to make a low-priced product is to skimp on quality, and that’s what we see time and time again.”
A CVS spokesperson said in a statement that the chain, which has more than 9,000 stores nationwide, prioritizes “good manufacturing practices and ethical sourcing.”
They added that CVS brands “are designed to maximize quality and safety, perform as intended, comply with regulations and satisfy customers.”
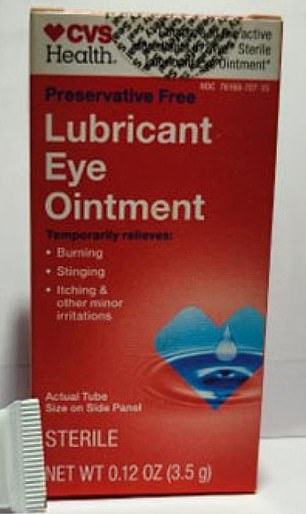

Shown above are two CVS brand products that were recalled due to contamination. They are eye ointments made in India and constipation pills made in Tennessee.

CVS said all of its brand-name drugs are manufactured to a high standard
In a case from January of this year, allergy medications made for CVS by an Indian company were recalled after FDA inspectors found stagnant liquid (growing yeast and mold) inside a food purification unit. air at the drugmaker’s facilities in India, reports show.
And in a flash from October last year, investigators arrived at a factory in India that made CVS eye drops and found peeling paint and barefoot workers. An FDA investigation also revealed fabricated test results that made factory products appear safe.
In a third case from 2022, CVS recalled magnesium citrate tablets (used for constipation) after they were found to be contaminated with microbes. The tablets were manufactured by the Tennessee-based company Vi-Jon.
In a fourth case from 2019, FDA inspectors found that a factory in Florida that made children’s cough syrup for CVS was using water contaminated with bacteria that can be deadly to young people with weakened immune systems.
Inspectors determined that the company, called Unipharma LLC and now defunct, had been ignoring test results that revealed the bacteria.
The discovery prompted a recall of all of its over-the-counter products. Also includes cherry-flavored kids’ pain relievers and fever relievers, mixed berry-flavored kids’ pain relievers, and pineapple-flavored kids’ cough syrup.
There was also a recall of CVS’s own-brand ibuprofen tablets that same year after researchers found that the pills contained more painkiller than the label suggested.
CVS sells more than 2,000 private-label health and wellness products in the U.S., and estimates the private-label market will be worth $236 billion by 2023.
It comes after the United States was rocked by the recall of more than 26 brand-name eye drops sold at pharmacies across the country, including Rite Aid and Walmart.
The drops were contaminated with a bacteria linked to blindness and fatal infections that experts said could “melt” through the eyes.
At least four people died of sepsis after using the eye drops, while 14 lost vision and more than 80 infections were reported.