Weight-loss injections such as Ozempic have been linked to 162 deaths in the US, DailyMail.com can reveal.
One of the victims was a 45-year-old woman who choked on her own vomit while taking Mounjaro, a rival drug that works in the same way.
Another case was that of a 23-year-old man who died from vomiting, nausea and tachycardia after taking Wegovy.
None of the deaths have been shown to have been directly caused by the shots, but experts say the reports indicate cases where they are suspected to have played a role.
The deaths were recorded in the FDA’s FAERS database, which is used to monitor the safety of drugs after they are approved and put on the market.
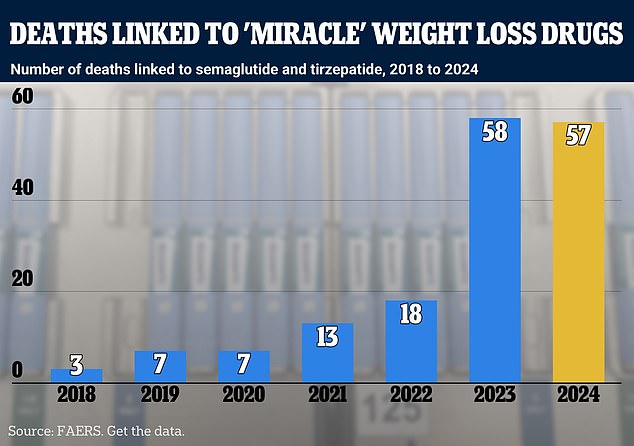
The chart above shows semaglutide and tirzepatide-related deaths by year. Semaglutide is the active ingredient in Ozempic and Wegovy, while tirzepatide is used in drugs such as Zepbound. Yellow is used for 2024 to indicate that the data is incomplete.
Deaths involving weight-loss drugs have increased by 40 percent in the past six months, when there were 117 deaths reported to the system.
The FAERS system also records adverse drug reactions, or when someone has a reaction after taking a medication.
Reports can be submitted by nurses, physicians, manufacturers, and patients themselves.
As with the deaths, the reports do not prove that a reaction was directly caused by a drug.
For example, someone might take a medication and then get food poisoning, which triggers stomach problems.
The system has recorded 62,000 reactions to weight-loss drugs containing semaglutide and tirzepatide, such as Ozempic and Mounjaro, in the US since 2018.
The vast majority of these cases (46,000) occurred after 2022, following a surge in use of the drug as more shots were approved.
Ozempic first became available in 2018, when it was approved for diabetics, but it was often prescribed off-label for weight loss.
Its sister drug, Wegovy, which uses slightly higher doses, was approved for weight loss in June 2021.
Eli Lilly’s drug Mounjaro, which uses tirzepatide, was approved for diabetics in May 2022, but has also been used off-label to help patients lose weight.
And in November 2023, the company’s Zepbound was approved for weight loss patients.
According to an analysis of data from healthcare firm Epic Research, about 1.7 percent of Americans (or 5.6 million people) will be prescribed a weight-loss drug in 2023.
That was 40 times higher than five years earlier, when it was estimated that only a few hundred thousand Americans were taking the drug.
This year’s surveys suggest that six percent of American adults (or 15.5 million people) have tried some form of weight-loss drug.
In FAERS, a total of 10,000 reactions were classified as “serious,” or in which the patient was hospitalized or suffered a life-threatening event.
Among the new cases reported in the past six months was a 30-year-old man taking Ozempic who was hospitalized with pancreatitis, a serious diagnosis in which the pancreas becomes inflamed and causes pain in the abdomen that some patients describe as “worse than childbirth.”
Her case was reported in June but it occurred in April of last year.
And in another case that occurred in May of this year, and was reported in June, a 49-year-old woman was hospitalized after experiencing mania and increased blood pressure while taking Ozempic.

Juanita Gantt was found unconscious by her husband Robert one day in October 2023 and rushed to the hospital to discover she had a severe case of colitis requiring removal of her colon.

Australian Trish Webster, 56, pictured above, died after using Ozempic to lose some weight before her daughter’s wedding.
In total, the FDA system has recorded 162 deaths among people who took blockbuster weight-loss drugs since 2018.
Of these, 94 were related to semaglutide, the active drug in Ozempic and Wegovy.
And the other 68 were linked to tirzepatide, which is the drug used in the weight-loss injections Mounjaro and Zepbound.
Of the 64 deaths recorded in 2023, 30 were related to semaglutide, while 32 were related to tirzepatide.
In 2023 and 2024, tirzepatide was linked to nearly twice as many adverse reactions as semaglutide, including a 48-year-old woman who experienced severe bleeding at the injection site and a 26-year-old man who said he had lower abdominal pain.
In 2023, there were 6,300 reactions related to semaglutide compared to 15,500 related to tirzepatide.
So far in 2024, there have been 4,710 reactions related to semaglutide, but 19,450 related to tirzepatide.
Dr. Adam Rubinstein, a plastic surgeon in Miami, Florida, said he was “shocked” by the mortality figures, adding that he rarely hears of significant complications in patients taking the drugs.
“The only major complaint I’ve heard anecdotally is pancreatitis,” he told DailyMail.com. “I find these figures very surprising.”
He added: “Many of them probably have a fairly loose association with medication because this number seems quite high.”
He also said it was possible tirzepatide was causing more reactions than semaglutide because it was “slightly more potent” and was suggested to cause slightly more weight loss.
“If there is greater weight loss, then one would expect the side effects to be greater,” he said.
A woman in Pennsylvania came forward this week to reveal the near-death experience she suffered while taking Ozempic.
Juanita Gantt, a 62-year-old diabetic, had been improving her condition for months by taking the medication until she suddenly collapsed at home and was found unconscious by her husband.
Doctors discovered that part of his intestine had died due to a condition called ischemic colitis, so his colon had to be removed. He subsequently suffered cardiac arrest.
The woman now has to wear a drainage bag (a bag used to collect waste from the intestines when the normal route no longer works) for the rest of her life when she goes to the bathroom, which she says she would not have needed if she had never taken Ozempic.
Almost all FAERS reports have been submitted by the manufacturers, Novo Nordisk or Eli Lilly, who are required to report adverse events to the FDA. They are often reported to them by patients or health care professionals.
The FDA said it does not comment on third-party evaluations in FAERS, such as those conducted by the media.
An agency spokeswoman added: “Protecting patients from unsafe, ineffective and poor quality drugs is central to the FDA’s mission to promote and protect consumer health.
‘The FDA maintains robust postmarket surveillance and risk assessment programs for approved products to identify problems that did not appear during the product development process.
‘However, spontaneous reports of adverse events often lack the complete information necessary to make a reasonable inference about whether a relationship exists between a product and an adverse event.’
The agency added: “Duplicate reporting and increased awareness of an event with a particular product may inflate the reported occurrence of an adverse event.”
Compared with 100 deaths linked to weight-loss drugs each year, there are 16,000 attributed every 12 months to NSAIDs, nonsteroidal anti-inflammatory drugs used to treat pain and inflammation, such as ibuprofen and aspirin.
The FDA has previously warned that in rare cases Ozempic can cause an intestinal blockage, called ileus, because it slows the passage of food through the intestines.
This can cause them to rupture and spill their contents into the body, which can lead to sepsis and multi-organ failure if not treated quickly.
Many patients taking the drug are also obese, putting them at higher risk for serious side effects from any complications caused by the drug.