Doctors are warned that a life-saving cancer therapy can cause new tumors to form in rare cases.
In a report published in the New England Journal of Medicine, researchers urged doctors to “be on the lookout” for unusual symptoms in patients receiving CAR-T therapy.
Twenty-five people out of 30,000 in the United States have been diagnosed with a secondary cancer after receiving treatment for a different cancer.
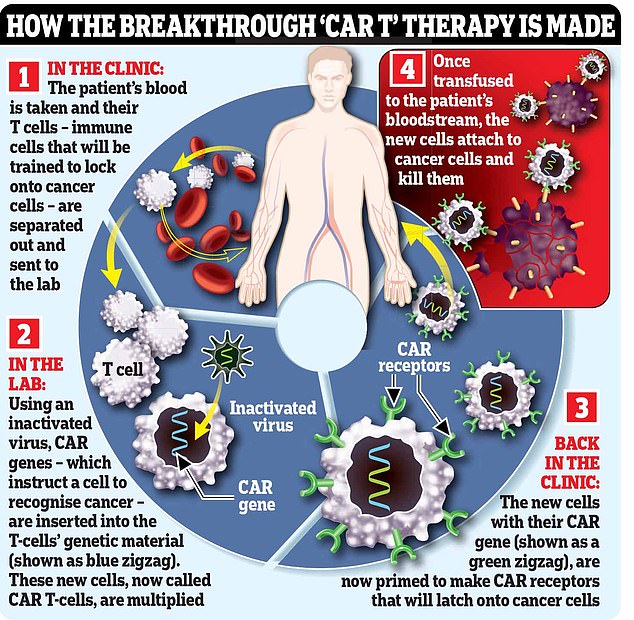
CAR-T, which was approved in 2017, takes immune cells from the body and engineers them to attack tumors before infusing them back into the blood.
CAR-T therapies, or chimeric antigen receptor T-cell therapies, were first approved in November 2017 and are reserved for cancer patients who would otherwise die without them.
But the way it is administered can alter cellular DNA and cause other cancers, posing a small risk in all so-called gene therapies.
This, the study authors emphasize, is a very rare scenario. According to their reports, more than 1 percent of people who have received CAR-T therapy have developed a secondary cancer from it.
But another new study from Stanford oncologists that was published in the same journal found that up to 6.5 percent of patients developed a secondary cancer within three years of receiving CAR-T therapy.
Even with these risks, CAR-T therapy saved many more people’s lives than it endangered, the authors of both new papers wrote.
It has been especially crucial in treating people who have not responded to any other therapy.
But as rare as this condition is, they want to raise awareness about it so that oncologists can watch for new cancer developments in their patients, said Dr. Metin Ozdemirli, a professor of pathology at Georgetown and co-author of the paper.
“When we know what to look for in advance, it’s easier to spot problems earlier,” Dr. Ozdemirli said.
This is especially prudent if trends persist and the therapy becomes more widely adopted. The FDA announced a review of the therapy in November 2023 to investigate 19 of these cases.
The NEJM report, published by oncologists and pathologists at Georgetown University Hospital, presented the case study of a 71-year-old woman who had been battling cancer for eight years.
He was given CAR-T therapy to treat myeloma, a type of cancer that affects the white blood cells in the bones.
After treatment, doctors ran tests and found no signs of cancer in his system.
Four months later, the patient returned to the doctor after suddenly losing 12 pounds and developing persistent diarrhea.
Doctors took a blood test and inserted a tube through the patient’s gastrointestinal tract to try to find the cause: a narrowing of abnormal cells in the intestine.
An illustration of a T cell, blue, attacking a cancer cell, red
He was initially diagnosed with an autoimmune disease and given steroids to treat the condition.
But when he didn’t get better, doctors biopsied a wound in his small intestine and discovered the truth: He had cancer again.
This time, the patient had developed lymphoma, which is a type of cancer that affects the body’s immune system, approximately nine months after CAR-T treatment.
When scientists analyzed the DNA of the new cancer cells, they determined that they had likely grown from the immune cells the patient was treated with to initially cure his myeloma.
Scientists still don’t understand how this happens, but they theorize that the cells they collected from the patient to perform the therapy in the first place could have had cancerous mutations. If that were the case, when the cells were placed in the patient, they could have turned into cancer.
The cells could also have mutated after being removed and before they were engineered into therapy, or after being reintroduced into the patient’s body, the case study explained.
Regardless of when the mutations developed, they caution that this rare side effect of the treatment should be something providers consider when using CAR-T therapy.
The patient has not yet overcome this new round of cancer, but as of April 2024, her symptoms have been improving slightly, the report details.