<!–
<!–
<!– <!–
<!–
<!–
<!–
Thousands of bottles of dry eye ointments sold at Walmart and CVS are being recalled over fears they could cause an eye infection.
Food and Drug Administration (FDA) inspectors ordered the recall after finding “unsanitary conditions” at the ointment factory in Maharashtra, western India, which they feared could mean the bottles were contaminated with microbes.
The recall is for 25 lots of ointments, and customers are encouraged to return them to stores for a full refund. To date, no infections have been recorded.
It comes after four Americans died after using eye drops laced with drug-resistant bacteria that were also supplied to the US from India.
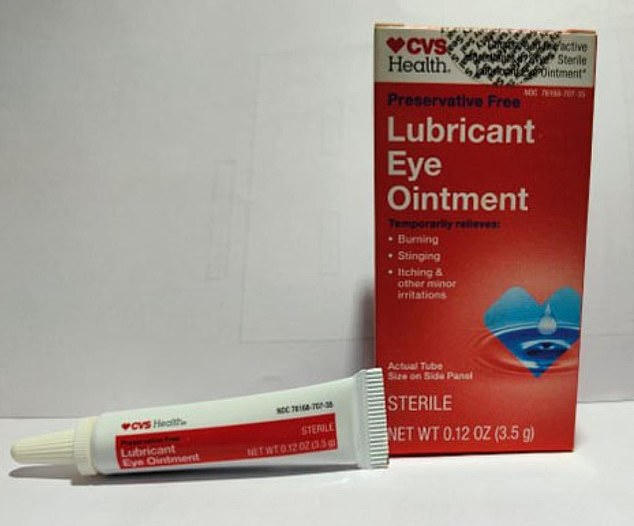
Eye drops being recalled include Lubricating Eye Ointment sold by CVS (pictured).
In the previous outbreak, four other people also suffered infections so severe that their eyeballs had to be removed, while 14 suffered vision loss and 81 reported eye infections.
Since then, the FDA has conducted inspections at factories across India and prompted recalls of eye drops or ointments from at least two other factories.
The latest recall is for four eye ointments sold in the US.— including Equate Lubricating Eye Ointment and CVS Health Lubricating Eye Ointment.
The FDA did not detail the “unsanitary conditions” that triggered the recall, but in previous cases, this was because workers were barefoot in factories or sterility test results were found to be retroactive.
The bottles recalled from the market were due to expire between April 2024 and September 2025.
It is not clear what microbes may be contaminating the bottles, but it was previously the bacteria Pseudomonas aeruginosa.
This is a particularly worrying microbe because it can “melt” the eyeballs and, in severe cases, can cause blindness and even death.
The FDA has published a list of the recalled LOT numbers, which can be found on the side of the product packaging.
Brassica Pharma manufactures eye drops and also a variety of other products, including cough formulas and multivitamin syrups.
Some ointments and eye drops, such as organic brands, are more vulnerable to contamination because they do not contain preservatives, which can keep microbes at bay.
In the recall notice, the FDA said, “For patients using these products, there is a potential risk of eye infections or related harm.”
‘These products are intended to be sterile.
‘Ophthalmic medications [eye drugs like eye ointments] They pose a potentially greater risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.
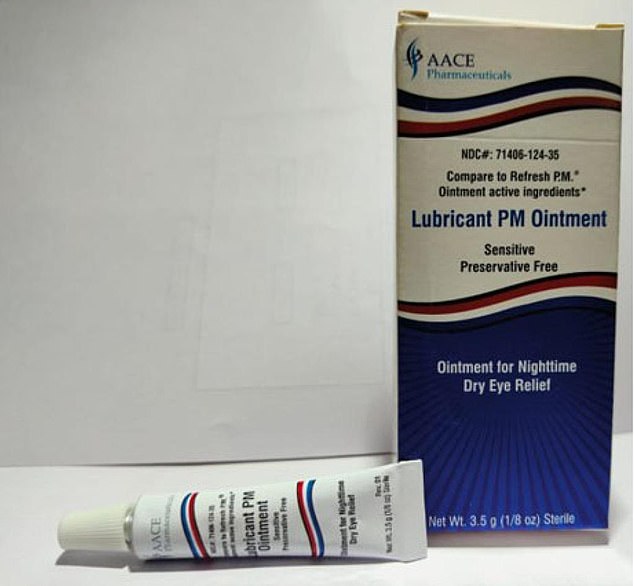

They also include an ointment sold by New Jersey-based AACE Pharmaceuticals.




And two Equate brand ointments that are available in Walmart stores (pictured)
They added: “Consumers should stop using the recalled eye ointment and may return.” [it] to the place of purchase.’
Eye ointments are used by people with dry eyes, including contact lens wearers and adults over 50 whose eyes produce fewer tears.
The FDA carries out inspections on the supply of medical products to the US market, including factories in other countries, such as India.

