It is one of the most discussed household chores, even at 10 Downing Street.
And with so many ways to stack a dishwasher, it’s often confusing to know exactly how to get the cleanest results.
Should you pre-rinse dishes? Does it make a difference if you put them on the top or bottom shelf? And how exactly should the cutlery be placed?
Now, MailOnline has spoken to engineers and appliance experts to find out the best way to load your dishwasher according to science.
Dr. Rainer Stamminger, a physicist and professor of household engineering at the University of Bonn in Germany, said it is not necessary to pre-rinse dishes, a mistake many households make.
From silverware placement to the best place for a cleaner wash, experts reveal the best way to stack the dishwasher


With so many ways to stack a dishwasher, it’s often confusing to know exactly how to get the cleanest results. Now, MailOnline has spoken to engineers and appliance experts to find out the best way to load your dishwasher according to science
“It’s not necessary because it uses a lot of water and energy if you do it with hot water,” he told MailOnline.
“Just throw leftover food in the trash with a napkin, knife or fork.”
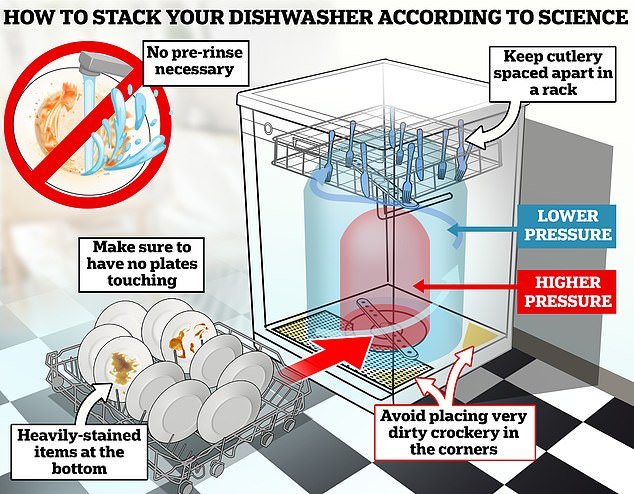
In general, heavily soiled items with stubborn food stains should be placed on the bottom shelf (where the rotating spray arms are located), because the water pressure is higher there.
This is also why delicate glass items or lightweight plastic containers should be placed on the top rack (where the pressure is lower), so that they do not break or get hit.
Also, avoid placing heavily soiled dishes in the four corners or on the shelves, as the water from the circular spray arm cannot reach them well.
“Don’t forget that water always comes up from the spray arms, so the dirty side should be facing down,” Dr. Stamminger said.
Some dishwasher users like to see how much they can fit at once, and while there’s nothing wrong with this, it’s important to make sure the dishes don’t touch.
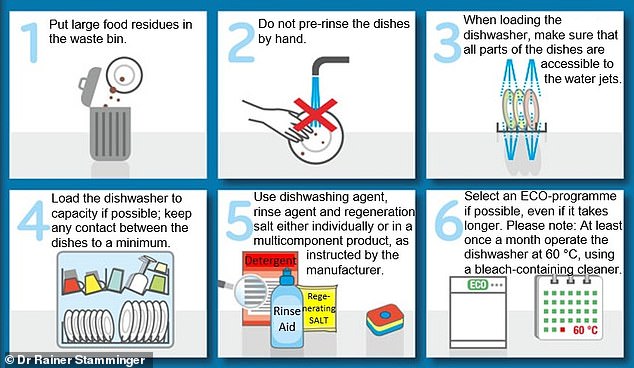

This graphic presents Dr. Rainer Stamminger’s six-step process for cleaning dishes in the dishwasher.
“There must be space between objects so that water can pass through,” Dr. Stamminger added.
Kiwi Services suggests leaving 1 to 2 inches of space between dishes to ensure adequate space for detergent and water circulation.
In 2015, a study led by an expert at the University of Birmingham suggested a radical arrangement of dishes in the dishwasher.
He said the most efficient way to pack dishes is in a circle, but with carb-stained plates in the center and protein-stained plates around the edge.
Water travels faster in the center, which is necessary to remove carbohydrate-based stains, the study explained.
Dr Stamminger told MailOnline that he disagrees with this recommendation, although he called it “very interesting”.
“All heavy and stained loads should be loaded near the center, at least in the area well reached by the water jets from the circle of rotation of the spray arm,” he said.
“The edges will only receive sparse splashes of water.”
Although most dishwashers have a basket on the bottom rack for stacking cutlery vertically, other models have racks right on top that stack cutlery horizontally.
Dmitry Letsman, Hotpoint dishwasher category manager, said the rack on top helps prevent dirty cutlery from sticking during the wash cycle, ensuring a thorough clean.
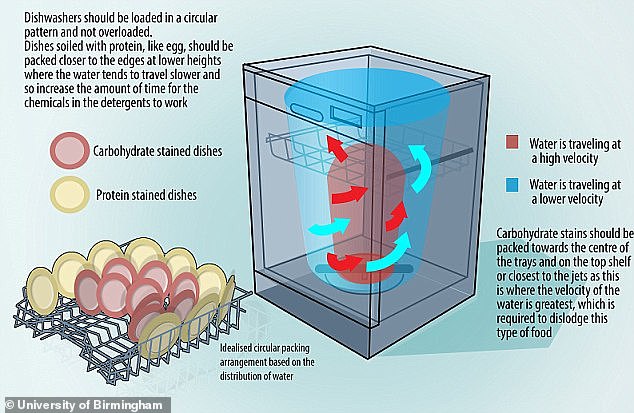

In 2015, engineers tracked the movement of water inside dishwashers before concluding that dishes should be arranged in a circle, but with carbohydrate-stained plates in the center and protein-stained plates around the edge. Carbohydrate-based stains require the full force of water jets to clean, while protein-based foods require more contact with the chemical detergent.
However, according to the expert, when it comes to cutlery there is “no really right or wrong method.”
“Placing cutlery in the basket is usually preferred for its space efficiency, especially for accommodating larger utensils such as serving spoons or tongs,” Letsman told MailOnline.
However, he urged the public to never put sharp knives, especially expensive chef’s knives, or wooden utensils, fine china or delicate glassware, into the machine.
“Over time, dishwashers can dull the blades of sharp kitchen knives, causing them to chip or rust,” Letsman added.
‘Handwashing and drying immediately is the best way to clean them.’
If you stack table knives vertically in the basket, for safety reasons, Dr. Stamminger recommends placing them upside down, that is, with the handle pointing up.
“Whether the cutlery is pointed up or down doesn’t have much impact on cleanliness, but when small children are around, it may be wise to turn it upside down,” he said.
