San Francisco voters turned away from their progressive reputation Tuesday night, when a series of ballot measures aimed at curbing crime and drug use overwhelmingly won the vote of city residents.
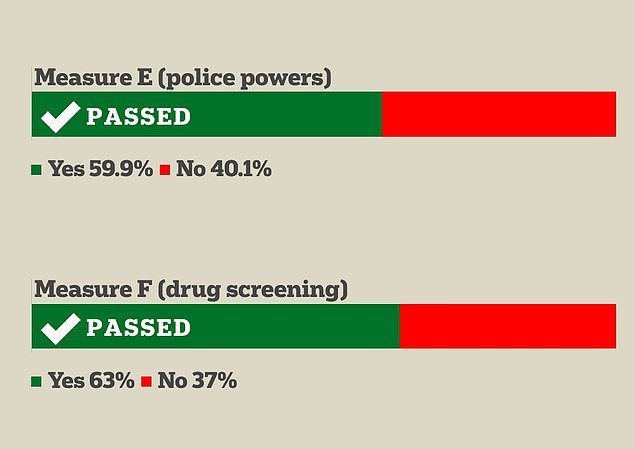
San Franciscans voted in favor of two measures, Measures E and F, which will respectively expand some police powers in the city and require drug testing for adults on welfare.
Proposition E, among other things, will make it easier for police officers to pursue suspects. It will also provide the force with new cameras and drones aimed at making their daily crime-fighting work easier and more effective.
And Proposition F is designed to help adults struggling with drug addiction by telling them that they will only remain eligible for cash welfare from the city if they enroll in a treatment program. Drug testing will be required to ensure compliance.

San Francisco Mayor London Breed scored a political victory Tuesday night when two ballot measures she supported passed: She’s in the midst of a tough re-election campaign against moderate candidates who say she hasn’t done enough to clean up the city.

San Francisco Mayor London Breed endorsed each measure on the ballot, calling it a victory. The Democrat herself has pivoted back from extremely progressive positions, including defunding the police, and her re-election is widely believed to be in jeopardy in the city’s mayoral election in November.
‘It’s clear that people want to see changes in public safety. The exciting thing about this for me is that I get the kind of tools I need to continue the work we’re doing,” said Breed, who is up for re-election in November.
When the results came in Tuesday night, Breed thanked voters for approving the measures. Prop E, he wrote, gives “our officers more tools to do their jobs.”
While Proposition F will be used to ‘bring more treatment and accountability to San Francisco.’ This is how we get more people the help they need and change what’s happening in our city.’
A spokesperson for Breed he told fox that the election results indicate that residents are “fed up and want more measures to tackle crime.”
‘Over the last few years, the city’s policies have leaned too far to the left. “Now is the time to send the message that San Francisco is closed to criminals and brazen theft will not be tolerated,” he said.
Breed faces an uphill battle to maintain his position. San Francisco voters are increasingly fed up with the city’s lax attitude toward drugs and violent crime.
Last year, more than 800 people in the city died from accidental drug overdoses; many of them were addicts living on the city streets, plagued by large amounts of fentanyl and xylazine.
Breed’s campaign, to some extent, has read the tea leaves and is trying to shape a campaign around a more moderate platform that takes into account the quality of life for tax-paying, law-abiding San Franciscans. .
For many years, the city has voted for hyper-progressive politicians and measures, but the tide began to turn in 2022, when voters remembered far-left district attorney Chesa Boudin, known for allowing violent criminals back on the streets. after his arrests.


Proposition E, which passed by 20 points, will allow police officers to more easily pursue suspects. It will also provide new drones and cameras to the force allowing them to improve their practice.


Starting in 2022, Mayor Breed has promised to crack down on the city’s open-air drug market and allow police to arrest people who inject in broad daylight.
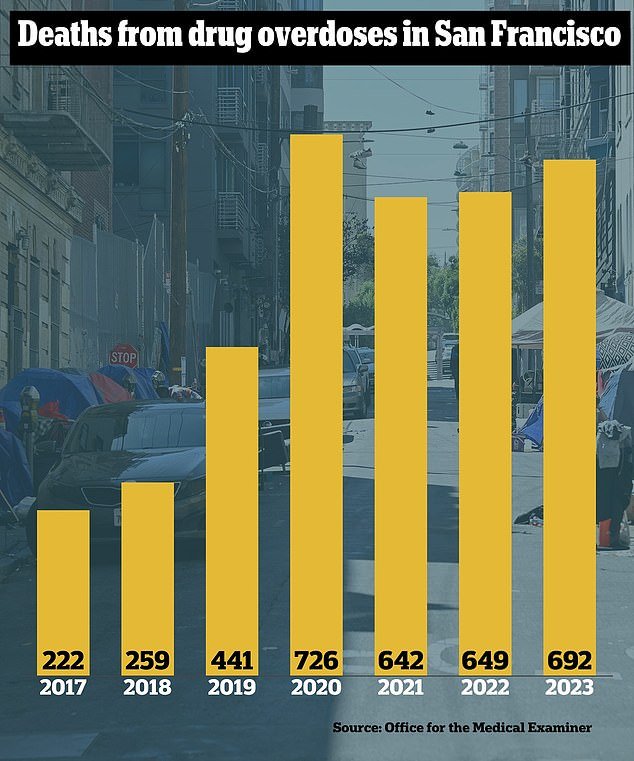

Last year, San Francisco was on track to have its deadliest year ever for drug overdoses: 692 people died by November and the total was forecast to reach more than 800.
Opponents of the proposals said they will be harmful to privacy and civil liberties and will only harm the marginalized communities the city is proud to serve.
But Breed, the first black woman to lead San Francisco, said at a campaign event in January that residents of poorer, black and immigrant neighborhoods are asking for more police.
Leaders of nonprofit organizations that primarily work with low-income people support Breed’s drug testing measure.
Trent Rhorer, executive director of the San Francisco Human Services Agency, which provides cash assistance and employment services to low-income residents without dependent children, said the current situation in the city is not improving lives.
“Giving someone addicted to fentanyl $700 a month, I don’t think it’s going to help improve their lives,” he said. “In fact, I think it does the opposite.”
Rhorer said the single adult welfare program, which serves about 9,000 people a year, already asks applicants about substance abuse, and about 20 percent report a problem.
A data check with the Department of Public Health revealed that nearly a third of recipients have been diagnosed with a substance use disorder, he said.
The ballot measure would replace that question with a more rigorous screening test that would be verified by an addiction specialist.
If substance abuse is discovered, Rhorer said, the specialist and the applicant will agree on treatment options that include residential care, a 12-step program, individual counseling and replacement medications.
The person doesn’t have to be sober, just make good faith efforts to attend your program, with the hope that “at some point a light bulb will go off,” Rhorer said.
The measure requires the city to pay rent for those accepted into the program for 30 days or more to avoid eviction.
About 30 percent of people who suffered a fatal overdose in 2023 were homeless, and more lived in subsidized urban housing.
