People who stop taking the weight loss drugs Ozempic and Wegovy are likely to face serious health problems.
A new study found that most people who stopped taking semaglutide, the active ingredient in the blockbuster drugs, regained about two-thirds of their lost weight and worse health markers, including higher blood pressure and cholesterol and a increased risk of heart disease.
Wegovy and Ozempic are known as GLP-1 receptor agonists and lead to weight loss by mimicking the actions of GLP-1, a hormone in the brain that regulates appetite and feelings of satiety.
About 12 percent of American adults report ever using a GLP-1 receptor agonist, while six percent indicated they were currently using the therapy.
About 85 percent of people who had recently started taking medications like Ozempic stopped using them within two years, and 71 percent stopped within the first year, either because they had reached their ideal weight or because the medications are Too expensive, priced at about $1,000. monthly.
Since it exploded onto the market, semaglutide has not only shown success in treating diabetes and obesity, but new research shows it also shows great promise in reducing blood pressure and the risk of heart attack or stroke. stroke.
Now, a study by Northwestern University researchers reported, while it is unclear how discontinuing GLP-1 RAs affects long-term heart health, the findings suggest it may increase cardiovascular risk.
But, given the high discontinuation rate and the bad side effects that follow, researchers said it’s essential to study whether taking these drugs in the first place offers any lasting benefits and decreases people’s health risks, or if the downsides of stopping medications outweighs the benefits.
A national survey found that about 12 percent of Americans have used one of these medications at some point, while six percent are currently taking Ozempic or Wegovy.
While the researchers behind the latest study did not conclusively say that patients will need to take these drugs forever to stay healthy, their findings indicate that people using GLP-1 RAs for weight management and cardiometabolic improvements may require regular use. long term to maintain profits. .
Discontinuation was also linked to a similar return in patients’ levels of certain cardiovascular and metabolic health risk factors, which play a role in heart health and diabetes.
The researchers reported: ‘Nearly 30 percent of individuals discontinued semaglutide in the SELECT trial, with real-world estimates for GLP-1 RA discontinuation in the range of 50 to 75 percent at 12 months.’
‘It is essential that clinicians and health systems identify and implement strategies that combine equitable initiation strategies with personalized support for the persistence of GLP-1 ARs. This requires understanding the underlying reasons for treatment discontinuation (with GLP-1 receptor agonists).’
The report was published in JAMA.
Previous research has found that after 68 weeks of injections, the average patient lost more than 15 percent of their body weight.
However, within 12 months of completing treatment, around 300 patients recovered approximately two thirds of the weight they had lost.
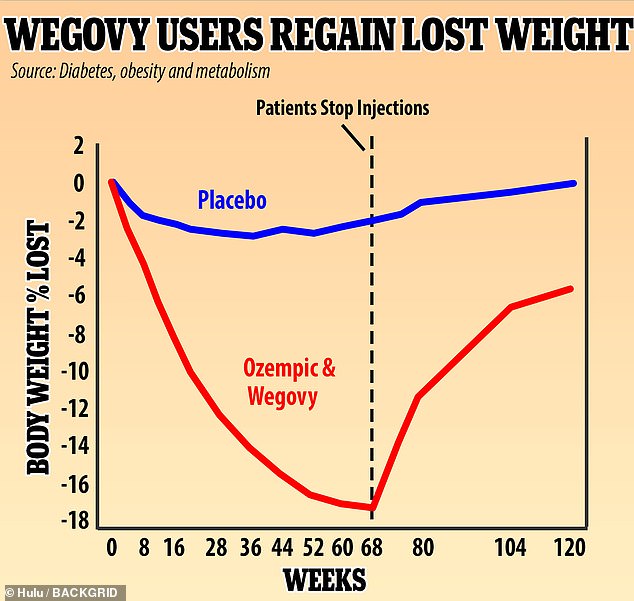
A UK study found that people who used Wegovy experienced rapid weight loss, losing 18% of their weight in 68 weeks. They regained two-thirds of that weight, or 12% of their original body weight, in the year after stopping the weekly injections. Experts say the drug should be used throughout life to avoid weight loss
The researchers added that doctors and patients should also discuss what might happen if therapy is stopped.
Dr Domenica Rubino, director of the Washington Center for Weight Management and Research, told the BBC: “Obesity is not like an infection where you take antibiotics and that’s it.”
“It’s no different than hypertension or diabetes or the many other chronic diseases we face, where you have to use chronic medications,” such as statins, for heart health.
While people who stop medications may experience undesirable results, there have been numerous reports of dangerous side effects in people actively using semaglutide, such as severe nausea and vomiting, suicidal thoughts, and stomach paralysis.
The introduction of Ozempic, first approved by the FDA to manage diabetes, and its sister drug Wegovy, now approved for weight loss, marked a seismic shift in the way doctors treat obesity.
The medications have been shown to help people lose between five and 20 percent of their body weight. The widespread use of these drugs has already influenced national obesity rates, which have fallen nearly five percent in one year.
Five million Americans were prescribed semaglutide in 2023, and nearly four in 10 were taking it specifically for weight loss, not diabetes management.
The researchers concluded: ‘Uptake of GLP-1 RA is likely to continue to increase rapidly in the US as coverage indications expand. But the staggeringly high discontinuation rates of GLP-1 RAs should raise alarm bells among doctors, policymakers and public health experts.’