The US FDA has approved donanemab, a treatment for Alzheimer’s disease that helps the body remove the characteristic plaque that builds up in the brain and causes dementia.
Manufactured by Indianapolis-based Eli Lilly, donanemab is a monoclonal antibody treatment designed to slow the progression of early signs of Alzheimer’s.
Eli Lilly said the drug, which has already been approved, will be sold under the name Kisunla and administered through intravenous infusions once a month. The cost will be $695 per vial, equivalent to about $32,000 a year.
While it represents a major advance in the treatment of Alzheimer’s, experts have raised concerns about donanemab because of the known risks of brain bleeding, which has killed several patients in the trial.
Manufactured by Indianapolis-based Eli Lilly, donanemab is a monoclonal antibody treatment designed to slow the progression of early signs of Alzheimer’s disease.
Donanemab will be sold under the name Kisunla and will cost $695 per vial, equivalent to about $32,000 a year.
In trials, donanemab was shown to only marginally slow the rate of progression and has dangerous side effects such as brain bleeding.
While the drug was hailed from the start as a potential therapy, a quarter of patients in the trial suffered brain inflammation and three people died from brain swelling or bleeding attributed to the drug.
In a press release, Eli Lilly said the drug slowed cognitive and functional decline in patients by up to 35 percent compared with placebo over 18 months.
It also reduced patients’ risk of progressing to advanced stages of Alzheimer’s by up to 39 percent.
As the U.S. population ages, so will dementia rates. Currently, an estimated 6.7 million Americans have Alzheimer’s disease (the most common cause of dementia), the vast majority of whom are over age 65.
It is estimated that by 2050, this figure will increase to almost 13 million.
Although the underlying cause of Alzheimer’s disease is still debated, scientists believe the damage likely results from an abnormal buildup of proteins (amyloid and tau) in and around brain cells.
In Alzheimer’s patients, amyloid proteins are not removed from the body efficiently and end up forming plaques in the brain. Tau proteins break off from neurons and form tangles.
Both can cause neurons to die, making it difficult for signals to be sent throughout the brain.
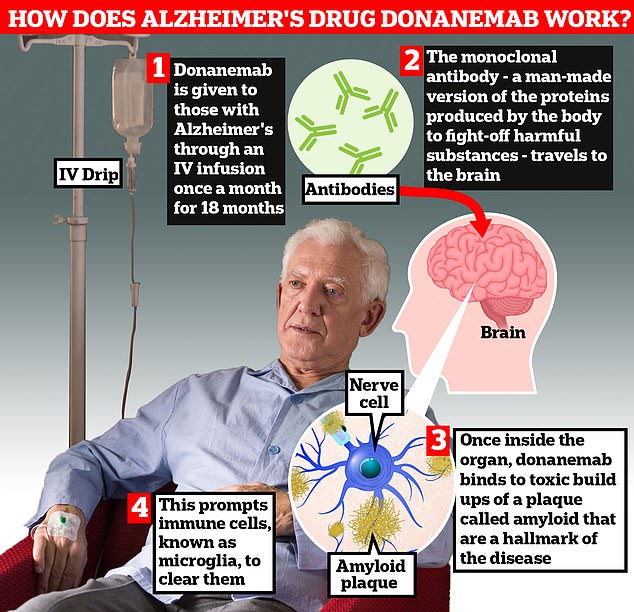
Donanemab is infused into the body intravenously and travels to the brain.
Once inside the organ, donanemab binds to toxic accumulations of amyloid plaque, which It prompts immune cells, known as microglia, to eliminate them.
Patients stop taking donanemab once the amyloid buildup has cleared from the brain.


