Two wonder drugs could help combat an explosion of colon cancers in young people, and one is already approved in the United States.
Both treatments are immunotherapies, which use patients’ own disease-fighting white blood cells to attack cancerous tumors.
Trials found that pembrolizumab, sold under the brand name Keytruda, “melts” tumors, potentially preventing patients from needing surgery and chemotherapy.
It was so effective that tests found that six out of 10 patients had no trace of disease months later. The drug is already used to treat lung and cervical cancer in the United States.
Meanwhile, a second new drug made by GSK was effective in 100 percent of cases of a rare form of colon cancer.
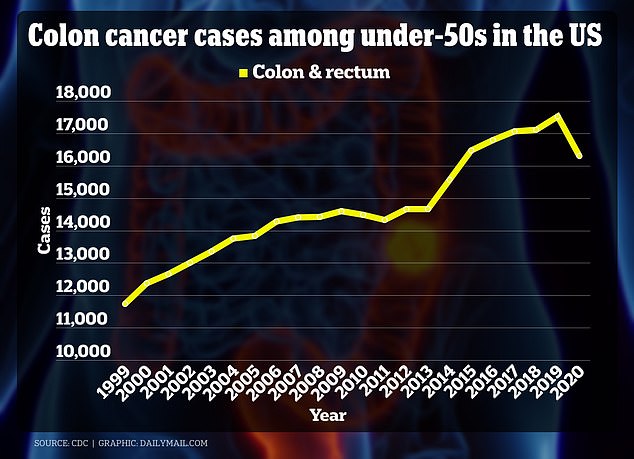
The graph above shows the increase in colorectal cancer in young Americans from 1999 to 2020.
The findings were presented this weekend at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
They come as colorectal cancer rates are expected to double from 2010 to the end of the decade.
Doctors are still trying to figure out what’s behind the rapid rise.
Processed foods, chemical contamination and excessive use of antibiotics have been pointed out as possible factors.
For the Keytruda study, researchers at University College London, in England, recruited 32 patients from five hospitals in the United Kingdom with stage two or three with a genetic subtype of colon cancer, with a high number of mutations.
The patients had stage 3 cancer, which meant the cancer threatened to spread outside the colon, which currently kills one in three within five years.
They received three doses of Keytruda over nine weeks before surgery.
The medication is administered as a 30-minute injection into the back of the hand and stimulates the body’s immune system to fight cancer cells.
After finishing the immunotherapy medication, the patients underwent surgery to remove the area of the intestine where their tumors had been.
The results showed that 59 percent of patients had no trace of cancer left when they were tested, usually between five and 19 months later, suggesting they did not even require surgery.
The remaining 41 percent could have their tumors removed and are now all free of the disease.
Doctors said this is a dramatic improvement compared to the current standard treatment, which involves surgery to remove the tumor followed by three to six months of chemotherapy.
Dr Kai-Keen Shiu, leader of the trial at the UCL Cancer Institute, said: “Immunotherapy can make tumors disappear before surgery.
‘If the cancer is melted before surgery, the chances of survival usually triple.
The drug is currently approved by the FDA for lung and cervical cancer, and is available on the UK NHS for cervical, lung, melanoma and triple-negative breast cancer.
The main limitation of the study was the small sample size. The team also noted that more research is needed with longer follow-up times in remission.
Additionally, immunotherapy has been shown to cost more than $100,000 without insurance, although it is often covered by private insurance and Medicare.
Keytruda has previously shown benefits in other cancers such as non-small cell lung cancer. In a testKeytruda after chemotherapy reduced the risk of disease progression by 42 percent compared to chemotherapy alone.
Additionally, findings presented at ASCO by Memorial Sloan Kettering in New York City found that the immunotherapy drug Jemperli, or dostarlimab, showed “unprecedented results” in eviscerating the colorectal cancers of all 42 patients.
All patients had locally advanced rectal cancer with mismatch repair deficiency (dMMR), a form of colorectal cancer that accounts for five to 10 percent of cases.
Of the 42 patients, 24 were followed up after approximately 24 months and none of the participants had disease recurrence. None of the patients required chemotherapy or surgery.
Hesham Abdullah, senior vice president at drugmaker GSK, said: “The data showing no evidence of disease in 42 patients is remarkable.”
“These results bring us one step closer to understanding the potential of dostarlimab in this curative-intent setting for patients with locally advanced rectal cancer with dMMR.”
Jemperli is currently approved by the FDA for endometrial cancer.