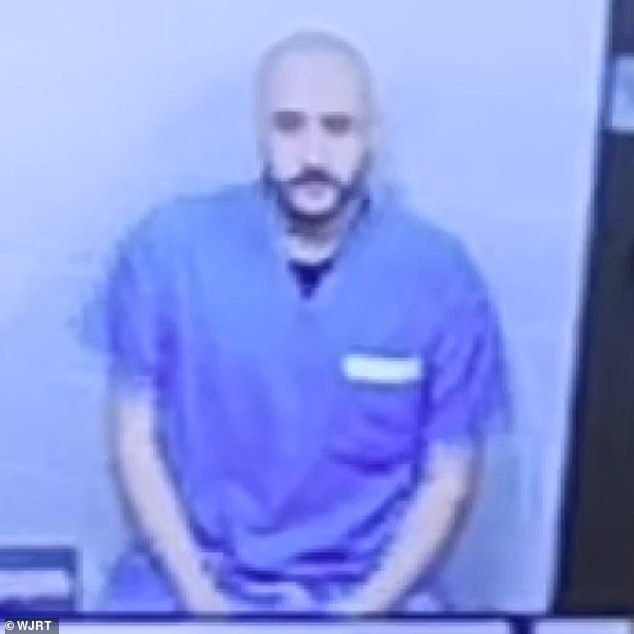
- Dr. Omar Najib Marar, 37, had threatened to “get his bomb belt and blow everyone up” in an operating room at Ascension St. Mary’s Hospital on January 8.
- The colorectal specialist was arrested on February 27 and charged with one count of falsely reporting a terrorist threat.
- His bail was set at $100,000 cash surety, which he posted and was released.
<!–
<!–
<!– <!–
<!–
<!–
<!–
A Michigan surgeon has been arrested for allegedly threatening to blow up a hospital after becoming frustrated over a malfunctioning CO2 tank.
Dr. Omar Najib Marar, 37, is accused of telling staff he was going to “take his bomb belt and blow everyone up” while in an operating room at Ascension St. Mary’s Hospital on Jan. 8.
The Saginaw Police Department arrested the colorectal specialist on Feb. 27 and charged him with one count of false reporting of terrorist threat, for which he could be sentenced to 20 years in prison.
During his arraignment in Saginaw County District Court, prosecutors said the incident was not isolated.
In December 2022, Marar went on a tirade while performing surgery, according to Saginaw County Deputy Prosecutor Melissa J. Hoover.

Dr. Omar Najib Marar, 37, was arrested after threatening to “take his bomb belt and blow everyone up.”


The blast occurred in an operating room at Ascension St. Mary’s Hospital on January 8.
He said he was going to kill himself because “staff were not performing to his standards,” court documents state.
Marar specifically stated that he would use a .22 or .25 caliber pistol to shoot himself and others in the operating room while continuing to perform surgery, Hoover wrote.
When staff informed seniors of the colorectal specialist’s behavior, he allegedly warned that he would “eliminate” those who turned him in.
Court documents claim many staff members were terrified that Marar would follow through on his threats.
His bail was set at $100,000 cash surety, which he posted and was released. He has pleaded not guilty in court.
As a condition of his bail, he is not allowed to have contact with three alleged victims. Authorities have not revealed the identity of these victims.
Marar has been associated with Central Michigan University Health since 2018.
He specializes in diseases of the colon and rectum, colon cancer, hemorrhoid surgery, Chron’s disease and ulcerative colitis.

In December 2022, Marar said he was going to commit suicide because “staff were not performing to his standards,” according to court documents.

His bail was set at $100,000 cash surety or 10 percent, which he posted and was released.
After her arrest, Mary Storm, a member of CMU Medical Education Partners saying: ‘Without commenting specifically on this case, CMU Health would like to share that the safety of our employees, patients and staff is our top priority, and we take any and all threats seriously. We also believe in a fair investigation and due process.
“However, in the event that any member of our staff or physicians exhibits behavior that is considered inappropriate or unprofessional, we take the measures we believe necessary at that time to prevent similar actions from occurring again through training, mentoring, counseling or other appropriate means based on the circumstances.’
The allegations were first examined by the FBI, but the investigation was soon turned over to the Saginaw Police Department.
He is scheduled to take a preliminary exam on March 20.
