- Patients with dementia are more likely to be infected with herpes simplex virus
- Between 50 and 80 percent of American adults are believed to have the cold sore virus.
- READ MORE: Doctors Demand Biden Take Dementia Tests After Week of Mistakes
People prone to cold sores may have twice the risk of dementia later in life, a study suggests.
Researchers at Uppsala University in Sweden found that people who had been infected with the herpes simplex virus (HSV), which causes cold sores, at some point in their lives were twice as likely to develop all forms of dementia. , compared to those who had never been. infected.
It is thought that the virus could increase the risk of Alzheimer’s because fragments of the virus remain in the body throughout life and there is some evidence that they travel to the brain, where they trigger the creation of beta amyloid and tau plaques, which are hallmarks of the illness. dementia.
Between 50 and 80 percent of American adults are believed to have the HSV virus. It remains dormant in the body, but at times when the immune system is weakened, exposure to the sun, cold wind, a cold or other illness, or even stress can cause outbreaks.
Researchers found that people who have been infected with the herpes simplex virus, which causes cold sores, at some point in their lives were twice as likely to develop dementia, compared to those who were never infected.
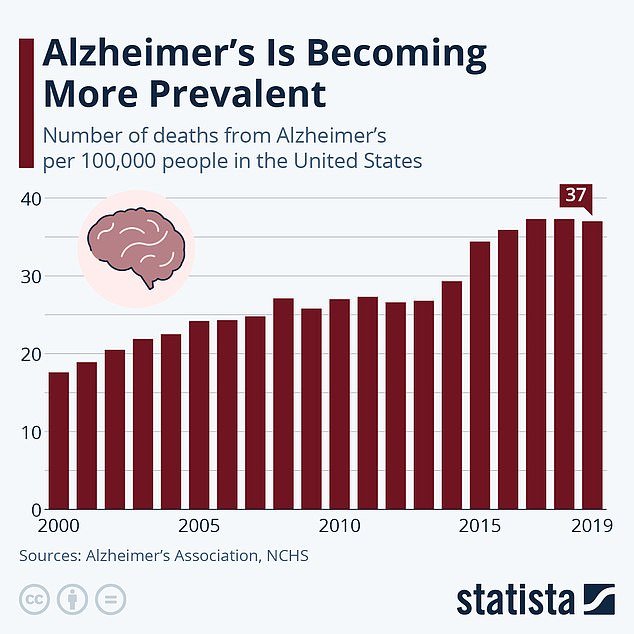
Alzheimer’s is increasingly common in the United States. The death rate from the disease has increased since 2000
While the study was observational and cannot prove the link, it aligns with previous findings.
For the latest research, published in the Alzheimer’s Disease JournalThe researchers studied more than 1,000 Swedes in their 70s for 15 years.
“What is special about this study is that the participants are approximately the same age, which makes the results even more reliable, since age differences, which are otherwise related to the development of dementia, cannot confuse the results,” explained Erika Vestin, a medical student. from Uppsala University, told Medical Xpress.
Blood samples were collected and tested for the herpes simplex virus.
The researchers also collected information on dementia diagnoses and indications of cognitive impairment from participants’ medical records.
Researchers at the Memory Clinic at Uppsala University Hospital reviewed the diagnoses and classified the cases as established or probable dementia.
About 71 participants (seven percent) developed dementia and 36 (four percent) developed Alzheimer’s.
About 89 percent of participants who developed Alzheimer’s or dementia had the herpes simplex virus, while 82 percent of those without cognitive impairment had the virus.
Using statistical analysis, the researchers determined that having the virus doubled the risk of dementia.
Dementia is the general term for a group of conditions associated with loss of memory, language and judgment.
Alzheimer’s is the most common form of the disease, affecting more than six million Americans, while Lewy body dementia is the second most common type, with approximately one million living with the condition.