One in 20 patients are forced to wait at least four weeks for a GP appointment, damning figures show.
The number of patients facing long waits of a month or more has skyrocketed 38 percent in the past year: from 12.8 to 17.6 million appointments.
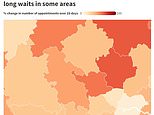
In parts of the country such as the Vale of York, four-week waits have soared by 80 per cent over the same period, according to an analysis of NHS data.
Meanwhile, more than 60 million consultations were made after waits of at least a fortnight, an increase of 22 per cent.
Your browser does not support iframes.
Your browser does not support iframes.
Patient groups said long waits are further evidence of “GP deserts” and warned the service risks “going down the same path as NHS dentistry”.
The analysis looked at the time between booking a GP appointment and the date it took place, broken down by local NHS areas across England.
All local areas in the country saw an increase in GP waits of four weeks in 2023 compared to the previous year, according to research by the Liberal Democrats.
This means that one in twenty (5.1 per cent) of the almost 348 million GP appointments last year involved waits of four weeks or more.
In some areas, including Gloucestershire, Derbyshire and Sheffield, South Yorkshire, almost one in ten consultations had waits of four weeks.
The Valley of York saw the biggest increase in GP waits of 4 weeks or longer, at 79 per cent, with 103,646 in 2023 compared to 57,779 in 2022.
Similarly, two-week waits increased 40 percent, from 263,758 to 369,231 during the same period.
Bury in Greater Manchester saw a 74 per cent increase in four-week waits, East Leicestershire and Rutland saw a 69 per cent increase and Blackpool, Lancashire, a 68 per cent increase.
In North Yorkshire, the Prime Minister’s backyard, there was a 56 per cent increase in four-week waits for GPs compared to the previous year.
Dennis Reed, director of over-60s campaign group Silver Voices, said: ‘The situation over access to GPs is becoming a bad joke.
“Patients experiencing anything but mild symptoms cannot endure a four or five week wait to see a GP.”
The situation is forcing people to avoid their GP, he said, often going straight to A&E, paying to go private or trying to treat themselves over the internet.
He added: “We are already seeing GP deserts developing, as this research shows, and we will soon see a situation similar to NHS dentistry, where it is almost impossible to access an NHS GP.”
A survey by the King’s Fund last week found that only a third of people are satisfied with GP services, the lowest level in more than four decades.
Your browser does not support iframes.
Long waits for appointments were one of the key reasons given for poor ratings, with satisfaction with GP services falling 34 percentage points from 2019.
The NHS says it is offering more GP appointments than ever and some non-urgent appointments, such as vaccinations, are being booked further in advance.
Dr Victoria Tzortziou-Brown, vice-president of the Royal College of GPs, said many appointments made several weeks in advance are entirely appropriate, for example for routine or review appointments.
“But we are concerned that many patients have difficulty seeing their GP and do not have enough time with us when they do get an appointment,” he said.
“The bottom line is that we don’t have enough GPs to meet the growing need for our care, and patients are feeling the impact most.”
The average number of patients per fully qualified GP is now 2,298, meaning each GP is, on average, responsible for 158 more patients than five years ago, he added.
A Department of Health and Social Care spokesperson said: “We are committed to improving access to GPs and, thanks to our plan for a faster, simpler and fairer health system, we are now providing 50 million more GP appointments per year”.
‘Our Primary Care Recovery Plan, backed by £645 million over two years, marks a significant investment in primary care services. This includes expanding the services offered by community pharmacies through Pharmacy First, which will help free up up to 10 million GP appointments per year.
“We are also investing £240 million in digital tools, telephony and training to ensure GP surgeries have what they need to improve patient access.”