Parents of UC Berkeley students raised $40,000 to cover the cost of private security near the university following a campus and citywide initiative to defund the police.
Safe Bears, a nonprofit made up primarily of concerned parents, raised funds for a two-and-a-half-week pilot program in which security guards in fluorescent jackets roamed the campus.
Unarmed officers staked out several blocks around the university from March 6 to March 23, operating separately from the UC Berkeley Campus Police Department.
The program came on the heels of a spike in crime in the city that saw a massive push to reduce police presence during the Black Lives Matter movement.
Speaking to NBC Bay Area, Sagar Jethani, president of Safe Bears and father of two students, said the program had received “positive feedback.” He and other members of the organization hope to see more private security hired in the future.
“Everything went very well, we brought a total of six security ambassadors, some were deployed on foot and others on bicycles,” Jethani explained.

Parents of Cal-Berkeley students raised $40,000 to fund a two-week private security pilot program after a spike in crime near the liberal campus.


The unarmed guards patrolled a stretch of blocks surrounding the university between March 6 and 23.


The decision came after a years-long effort by the student body to defund campus and city police.
“They provided escorts to students who needed them, security escorts, gave instructions and were also a very visible deterrent against crime.”
UC Berkeley experienced an increase in crime between 2021 and 2022. Notably, the number of aggravated assaults increased from 54 to 63.
Parents claimed that university police were understaffed and overwhelmed by the increasing violence.
Their concerns came to a head in 2022, when, following an altercation a block from campus, a student was shot and killed.
Police said the shooting stemmed from an earlier fight. Three men aged 22, 24 and 28 were injured, while Isamaeli Mataafam, 29, died.
Mataafam was a doctoral student at the Pacific School of Religion at the time of his death.
According to Jethani, the Safe Bears program was inspired by the private security UC Berkeley hired after the shooting. Private security provider Streetplus was chosen for the job due to its existing relationship with the city.
Last month, another shooting occurred on campus when Virgil Hampton, 59, allegedly fired his gun after a “physical altercation” with a student, the University of California Police Department said.
The man ordered the students to open their backpacks and give him a charger. When a fight broke out, he pulled out a gun and started shooting into the air.
While the explosion only shattered a window, it left the community shocked.


Sagar Jethani, president of Safe Bears, a nonprofit organization made up primarily of parents, said the pilot program had received “positive feedback.”
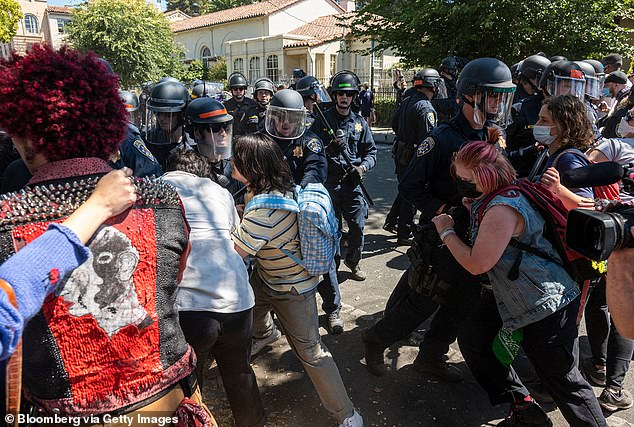
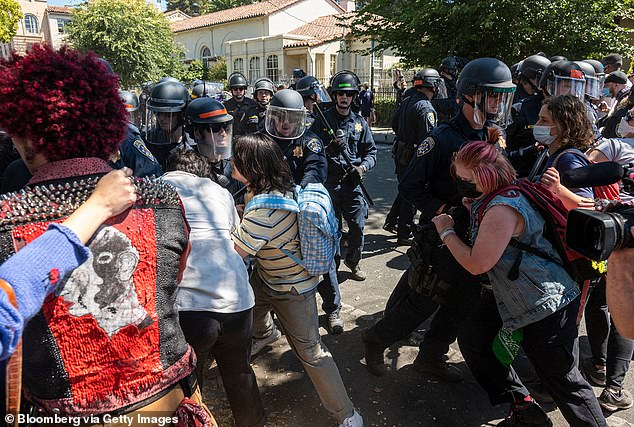
Student organizations, including the Black Student Union, have argued that Cal-Berkeley “plays an active role in this abusive system” by partnering with city police and funding campus police.


Meanwhile, parents have insisted that university police are overwhelmed by rising rates of violent crime.
The increased security presence also comes after years of efforts to defund the police.
UC Berkeley has been a bastion of liberal thought in the Bay Area for decades, and the Princeton Review characterized the student body as generally “politically liberal, non-religious, and fairly independent.”
Calls to cut funding by university police arose from two separate incidents in 2019, which began when campus police responded to a report of two people on campus, one of whom was carrying a stun gun.
According to a notice from the school’s Black Student Union, two freshmen were walking with a University of San Francisco student in March when officers approached them and asked them if they had a gun.
The USF student admitted to carrying one for self-defense before one of the UC Berkeley students was “thrown to the ground, arrested and taken to UCPD for questioning.”
The University of San Francisco student was also arrested. Both students were cited and released, but neither was read their rights during their arrest, the union said.
In a subsequent incident in June 2019, campus police detained two black children – children of UC Berkeley students – aAfter they called the police to report that a stranger was taking photos of them.
In a demand letter sent to the institution, a coalition of student groups alleged that police also “used excessive force against the children” when they put them in the back of a police car.
School spokeswoman Janet Gilmore later admitted that there was “no further need for police action” at the time, even though an 11-year-old boy was handcuffed.
“By continuing to partner with Berkeley PD and fund UCPD, UC Berkeley plays an active role in this abusive system,” the letter said.
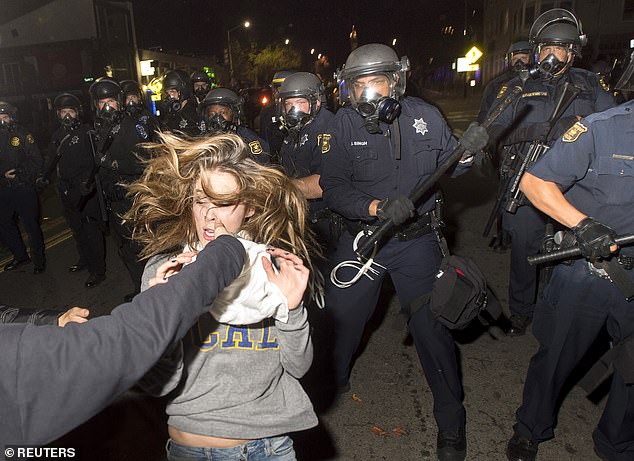
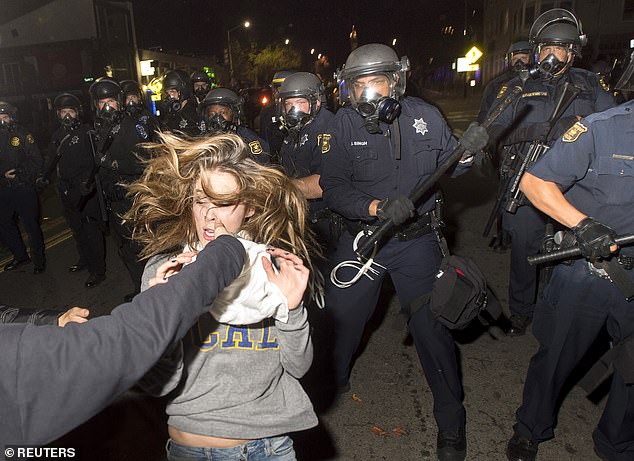
A protester near Berkeley flees as police officers try to disperse a crowd made up mainly of students during a protest against police violence in the United States.


The charge to defund police on campus mirrored similar calls in the city of Berkeley, where the City Council voted in 2020 to cut $9.2 million from the police department’s budget.


In May 2022, City Council opted to ‘refund the police,’ shelling out $5.3 million for public safety programs and restoring 30 roles within the department.
The charge of defunding the police came against a backdrop of similar calls in the broader city.
In 2020, amid protests over the murder of George Floyd, the Berkeley City Council voted to cut the police budget by $9.2 million, which represented 12 percent of the department’s annual operating budget. Thirty positions in the police department were frozen.
At the time, city departments had to take cost-saving measures due to pandemic shortfalls.
Mayor Jesse Arreguín said the department would have reclaimed 50 percent of the city’s discretionary fund over the next five years if such action had not been taken.
In May 2022, the City Council opted to “refund the police” after reaching a compromise that awarded $5.3 million to public safety programs.
Several council members also pushed for the 30 frozen positions to be restored.
Arreguín, who had previously been a leading advocate of cutting funding, called the vote an “important milestone.”
The decision also came amid skyrocketing crime rates. During a March 2023 meeting, Interim Police Chief Jen Louis shared that the total number of violent and property crimes in 2022 was “the highest in the last 10 years.”
“Berkeley continues to have one of the highest property crime rates in our region,” he said at the time.
Assaults and assaults had increased 1 percent so far this year, with 148 reports so far. Reports of stolen vehicles saw the largest increase, increasing 12 percent with 293 reports. Meanwhile, robberies, sexual assaults and burglaries decreased.
City leaders have claimed that most of the department’s time is consumed by low-level calls, leading to less focus on violent crimes. One suggestion has been to move traffic enforcement away from police, which can also reduce racial disparities in policing.
