- Biden won the first round of races on Tuesday with the Iowa caucuses
- Trump seeks massive sweep of states
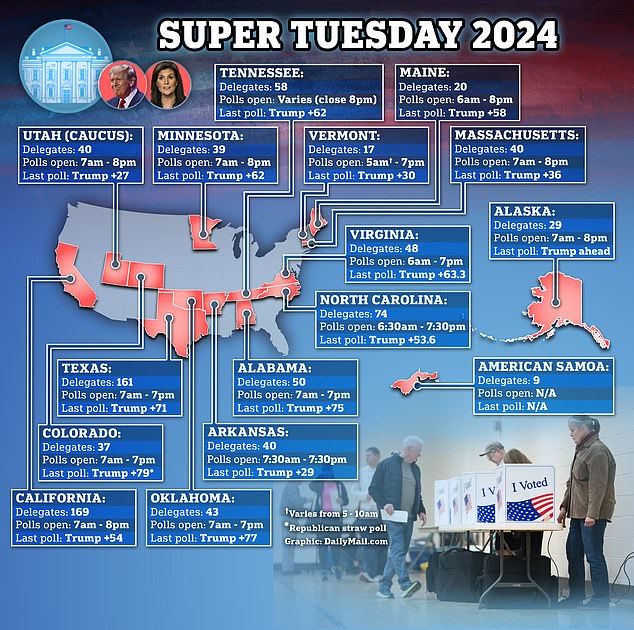
- 15 states hold nominating contests on Super Tuesday
<!–
<!–
<!– <!–
<!–
<!–
<!–
Virginia and Vermont became the first states to close their polls on Super Tuesday, the biggest day of the presidential primary season.
Fifteen states hold nominating contests. Millions of voters will cast their ballots and hundreds of delegates are at stake – the biggest haul for any candidate yet.
Subsequently, Joe Biden and Donald Trump are expected to move much closer to their parties’ respective nominations.

Voters cast their ballots at a voting center in Los Angeles County, California, on Super Tuesday.

Biden began the night by winning Iowa, where Democrats previously held their contest but released results on Tuesday.
Trump seeks to sweep the 15 states holding Republican primaries on Tuesday. He still faces opposition from Nikki Haley, but a series of victories by her will make it almost mathematically impossible for her to catch the former president.
Tuesday’s election will deliver more than a third of Republican delegates, and more than 70% of the number needed to secure the nomination.
Haley promised to stay in the race until the Super Tuesday contests, but she has not made any promises beyond that, and her campaign has not scheduled any public events for Tuesday or beyond.
Both the Biden and Trump camps have been preparing for a rematch even thoughThe earliest any of them can become their party’s presumptive nominee is March 12 for Trump and March 19 for Biden.
But after Tuesday, the battle for the general election will effectively be underway. Both men are ready.
“We have to beat Biden; he’s the worst president in history,” Trump said Tuesday on Fox & Friends.
“If we lose this election, you’re going back to Donald Trump,” Biden said on the DeDe in the Morning radio show in North Carolina. “I think the way he talks, the way he acts and the way he treats the African-American community has been disgraceful.”




Donald Trump (left) is hoping for a big victory on Super Tuesday that will knock Nikki Haley (right) out of the race.


Election workers process ballots at the Utah County election headquarters in Provo, Utah.
Voters, however, do not seem enthusiastic about the idea of repeating the 2020 election, which Biden won and Trump falsely claimed victory. Polls show that most voters wanted an option other than Biden or Trump.
So now each campaign will have to focus on rallying its base and persuading independent voters to go to the polls in November.
On Saturday, Biden and Trump will hold separate campaign events in Georgia, a critical battleground state. Biden became the first Democrat since Bill Clinton to win it in 2020. Trump was convinced he had won it and now faces federal and state charges for his attempts to overturn the results there.
