The maker of blockbuster weight-loss drugs has urged Hollywood stars to stop hoarding Ozempic and similar drugs because of shortages.
In a bizarre 30-second television ad, Eli Lilly, which makes the weight-loss drugs Mounjaro and Zepbound, urged those who are not fat to stay away from weight-loss drugs.
He said they had only been tested and approved for people who are overweight or obese (who are at higher risk of other conditions such as heart disease and cancer) or who have type two diabetes.
The blockbuster weight-loss drugs, which cost about $950 a month out of pocket, have been in short supply for months amid growing demand. Estimates suggest that up to nine million prescriptions for these medications were issued in the last three months of 2022.

The ad urged people who just want to lose a few pounds not to get prescriptions for weight-loss drugs.


Stars like Chelsea Hanlder, pictured above, have admitted to using the drugs.
The TV advert, called ‘Big Night’, begins by showing a sparkly dress on a bed, a red carpet and paparazzi.
It then cuts to show an overweight woman standing on the subway and clutching her purse.
One headline reads: “Some people have been using medications that were never intended for them.” For the smallest dress or tuxedo. For a great night. Out of vanity.
But that is not the point. “People whose health is affected by obesity are the reason we work with these medications.”
Company executives say they are showing the ad to ensure their drugs reach those who really need them.
The advertisement is broadcast on American channels just before the 96th edition of the Academy Awards, or Oscars, which will be held this Sunday in Hollywood, Los Angeles.
Last year, host Jimmy Kimmel joked about weight-loss medications, saying, “When I look around me, I can’t help but wonder: Is Ozempic right for me?”




Others who said they used weight-loss medications include Elon Musk and Amy Schumer.
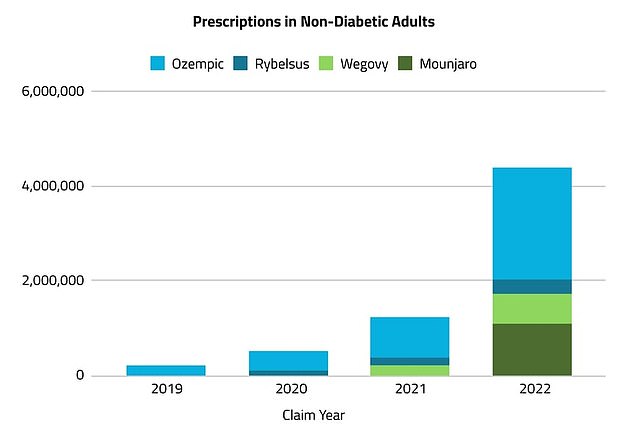

In 2022, more than 5 million prescriptions for Ozempic, Mounjaro, Rybelsus or Wegovy for weight management were issued, compared to just over 230,000 in 2019. This marks an increase of more than 2,000 percent, according to the research firm Komodo Health market.
Your browser does not support iframes.
At the time, a host of celebrities had revealed that they were taking the drugs, which remain in short supply, to help them lose a few pounds.
They included comedian Chelsea Handler, who said she lost several pounds on Ozempic, and Elon Musk, who said he lost nearly 30 pounds while taking the drug, and model Remi Bader.
Among the celebrities who have revealed that they have used Eli Lilly’s Mounjaro is Rosie O’Donnell, who said she was “very happy” with the results.
The number of prescriptions written for weight loss drugs in the U.S. has increased more than 2,000 percent since 2019, from 230,000 prescriptions in 2019.
Analysts predict the weight-loss drug market could reach a value of $80 billion by 2030, up from around $3 billion in 2022.
In many cases, doctors have been prescribing medications “off-label” to people looking to lose weight but who are not obese or have type 2 diabetes, helping to fuel the shortage.


The ad also showed someone spreading a dress on a bed, probably before an event.


And it showed hordes of photographers at the event.
Currently, the Food and Drug Administration lists four of five available doses of Wegovy, which is approved to help patients lose weight, as in short supply.
It also lists three of the six shots available for Mounjaro, which is approved only for type 2 diabetics, as in short supply.
The reason for this shortage is the increase in demand for the drug.
Eli Lilly CEO David Ricks said cnn: ‘We have a point of view on how these medications are used.
‘These medications were invented for people with a serious health problem; They weren’t invented just to make someone famous look a little better.’
Ozempic and similar medications have proven popular for their promise of helping people lose weight with nothing more than a weekly injection.
Studies showed that patients taking these medications lost 15 percent of their body weight, or about 30 pounds, over the course of 58 weeks.
But doctors are beginning to warn that the drugs must be prescribed carefully and that, if given incorrectly, patients can gain weight again later.
The medications work by mimicking a hunger hormone in the body, making someone feel full even if they have not eaten recently.
But doctors warn that patients should continue to eat a high-protein diet to avoid losing more muscle than fat.
This can cause problems because if they then go back to their previous diet, they will start to gain more weight than they lost because they now have less muscle mass.
