A billionaire heiress took revenge on locals who questioned her plans for her quiet Lake Superior neighborhood by reversing plans to modernize her community.
Kathy Cargill, the wife of James R. Cargill II, the patriarch of the fourth-richest family in the United States, has sparked controversy among residents of Duluth, Minnesota, after snapping up several homes in the area.
Cargill has been accused of tearing them down while refusing to reveal its plans, and not without offending locals, calling the houses “pieces of shit” to a local journalist.
But after residents raised the alarm and the new mayor demanded that Cargill reveal its intentions, she responded by canceling plans to build a sports complex and beautify the area.
“I think an expression we all know, don’t pee in your Cheerios, well, (the mayor) peed in his Cheerios right there, and I’m definitely not going to do anything to benefit that community,” he said. he said he to Wall Street Journal.
‘Whatever good plans I have there to beautify, update and spruce up Park Point Park or build that sports field, forget about it. There is another community with more welcoming people than that narrow-minded community.’

Kathy Cargill purchased several homes in Park Point, Minnesota, but canceled her plans to beautify and modernize the community after locals questioned her real estate spending spree.


One of the homes purchased by Cargill, 4202 Minnesota Ave (pictured), was purchased for $2.5 million and is being renovated.


Many of the homes have been purchased well above the market price. Cargill purchased 1223 Minnesota Av (pictured) for $350K, more than 100K above appraised value
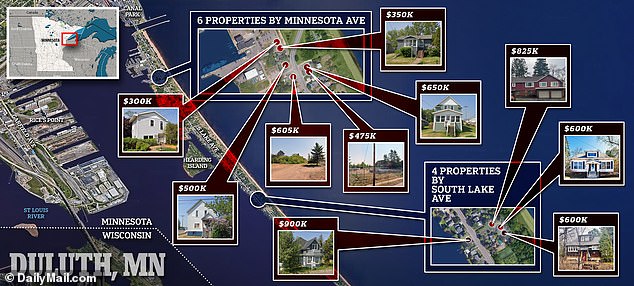

Ten of the homes purchased by Cargill look closely clustered in this chart, with a median price of around $477,000.
In total, Cargill reportedly purchased 20 homes and parcels of land in the area, acquiring many of them for well above market value.
“It’s so sudden,” Dawn Buck, president of the Park Point Community Club, told the Wall Street Journal.
‘What happens is the house closes, then immediately the stakes in the survey increase and within a week the utilities are cut off and, pretty soon, a bulldozer arrives. Someone somewhere has a plan.
In addition to its real estate spending spree, Cargill is also known for its love of McLaren supercars, which sell for more than $1 million each. Her net worth inherited from her husband is reportedly around $4.2 billion.
Rumors quickly spread through the community that Cargill intended to bulldoze the area to create a gated community for the super-rich.
Many were happy at the arrival of a billionaire who was looking to part with his money. But those who wanted to stay in their picturesque homes on Lake Superior feared it would threaten their taxes and the local housing supply, sparking a small riot in the sleepy town.
Led by the newly elected mayor of the city of 86,000, Roger Reinert, locals demanded to know what Cargill planned to do next.
“The plan for these properties is unknown, and that worries many, including me,” Reinert said on social media, while urging voters not to continue selling to Cargill.
This prompted Cargill to respond with its “pee in your Cheerios” taunt, while reversing its promises to fix the community.


Many of the properties have already been torn down and locals fear what will be built in their place. A vacant lot at 1314 Minnesota Ave, Duluth seen above


1239 Minnesota Av was purchased for $500 thousand, although it was appraised at $239.5 thousand


Park Point residents have expressed concern both about rising property taxes and why exactly the homes will be built on the site. Cargill purchased 1221 Minnesota Av for $300,000
He reportedly claimed he was still receiving calls from locals looking to cash in on their homes and did not rule out further purchases.
But he told the community they can “forget” about a cash injection for a sports complex or anything of the sort, while insisting he intends to keep a holiday home he bought in the area.
“We’re going to make it even more private than it is and still enjoy it,” he told the Wall Street Journal.
‘Those people are not kicking me out. You can take whatever stance you want, but I’m not going anywhere.
In December, Cargill sparked fury among locals when a journalist quoted her as saying: ‘The houses we bought were shit. I couldn’t imagine living in any of them.
Brooks Anderson, a 90-year-old retired minister, responded to the WSJ: “This is my shit and I love it.”
“I hope he regrets saying that.”
However, Cargill’s dispute with the community comes as some question why there was so much controversy in the first place, and say its new prominence in the area was a good thing.
Dan O’Neill, 71, who sold his $500,000 home to Cargill for $825,000, said: “I think Kathy Cargill is a well-intentioned lady. I have faith that they are going to do the right thing.”


2931 Lake Ave S, Duluth was purchased for $600K


Cargill reacted with fury after the mayor of the town of just 86,000 inhabitants demanded to know its intentions with the real estate purchases.
Cargill is known for its love of McLarens, which can drive at speeds in excess of 200 mph.
The Cargills are the fourth richest family in the United States, with an estimated net worth of $47 million.
His family now numbers 14 billionaires, more than any other family in the world.
Cargill was founded in 1865 by William Wallace Cargill. It is headquartered in Minnesota and is the largest agricultural company in the world, producing and processing agricultural products, but also offering a variety of financial services.
Most of the family members have extremely private lives, and according to Forbes, many live on ranches and farms throughout Montana.
There are very few photographs of the family in the public sphere. Duncan MacMillan’s 1998 book, “The American Grain Family,” described the family as “very stubbornly private.”
The company’s common stock has been owned by descendants of William Cargill and his son-in-law John MacMillan for more than 140 years and six members of the family sit on its 17-person board of directors.
The family is made up of almost 100 members who together own about 90 percent of the company. The remaining ten percent is owned by employees through stock ownership plans and management-owned stock.
