Fat loss injections such as Ozempic and Zepbound have been linked to more than 100 deaths in the US, DailyMail.com can reveal.
One of the victims was a person in their 20s who developed an “intestinal mass” and another was a pregnant woman.
The cases have been recorded in an FDA tracking system used to track the safety of drugs used in the US, called FAERS.
None of the deaths have been proven to have been directly caused by the injections. But experts say the reports indicate cases where drugs were suspected to be involved.
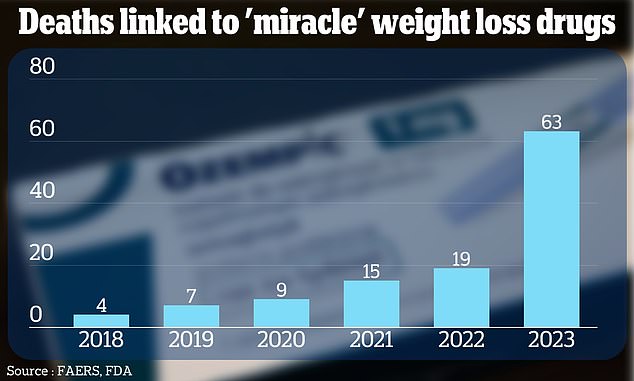
The cases have been recorded in an FDA tracking system used to track the safety of drugs used in the US, called FAERS. They are shown above in a graph.


Trish Webster, 56, pictured above, died after using Ozempic to lose some weight before her daughter’s wedding.
There are also concerns about counterfeit versions of the drugs, which are becoming more common as patients struggle to obtain the real drugs.
Fake versions have been found to contain insulin which, if not used in the correct doses, can cause seizures, coma and even death.
The youngest patient with information was a 28-year-old woman who was hospitalized and diagnosed with an “intestinal mass.”
Another death involved a woman who took Ozempic while pregnant, which the drug’s manufacturer advises against after studies of the drug in pregnant rats showed that their offspring had growth problems and developmental abnormalities.
In total, the FDA system has recorded 117 deaths among people taking blockbuster weight-loss drugs since 2018.
Of those, 81 were linked to patients using semaglutide (the active ingredient in Ozempic and Wegovy), while 36 were linked to patients using tirzepatide, the ingredient in Mounjaro and Zepbound.
More than half of the semaglutide-related deaths (54 deaths, or 66 percent) were recorded after a version of the drug, Wegovy, was approved for weight loss in June 2021. Ozempic has not been approved for lose weight, but it is prescribed without a prescription. label for this use.
No deaths related to tirzepatide have been reported after a version of the drug, Zepbound, was approved for weight loss but was not given the green light for this use until November 2023.
Symptoms recorded ranged from seizures to blockages in the intestines and pancreatic cancer.
Deaths increased 230 percent in 2023 compared to previous years, although experts said prescriptions also spiked around that time. If there are millions more people taking a drug, this increases the likelihood of deaths among patients who use it.
None of the American victims who died while using Ozempic have been identified.
However, last year, the family of a 56-year-old Australian mother said she had died from Ozempic, after she was diagnosed with “acute gastrointestinal illness” and collapsed at home with a “brown substance” foaming out of her. from the mouth.
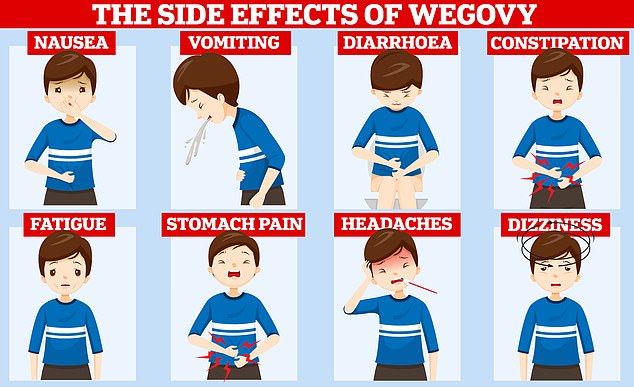

Despite being hailed as one of the most powerful pharmaceutical tools to date, experts have warned that it is neither a “magic pill” nor a miracle solution. Trials have shown that users can quickly gain weight again once they stop taking the medication and this can trigger a variety of unpleasant side effects. Users often complain of nausea, constipation and diarrhea.
He had lost 35 pounds (16 kg) while taking Ozempic and another weight-loss medication, while trying to lose weight for his daughter’s wedding.
Experts told DailyMail.com they were surprised that the number of deaths linked to weight-loss drugs was “not higher.”
Toni Adamrovich, a nurse and obesity medicine specialist at TB2 in Ohio, who works with patients prescribed semaglutide and tirzepatide, said: “I’m not surprised by this number. In fact, I’m surprised it’s not higher.
“I say this not because I think these medications are inherently dangerous, but because of the increase in their use for weight loss.”
He added: “Remember that the FDA Adverse Event Reporting System (FAERS) does not require a demonstration of a causal relationship between a drug and an event, so it may be ambiguous whether an event was due solely to the drug.” reported”.
“In contrast, the FDA also does not receive reports of all adverse events and any one death is too many.”
Broken down by drug, Ozempic had the most related deaths (56 deaths), followed by Mounjaro (36 deaths) and Wegovy, three deaths.
There were no deaths related to Zepbound (the active drug is tirzepatide), but there were 22 related to other semaglutide combinations.
Almost all FAERS reports were submitted by manufacturers (Novo Nordisk or Eli Lilly), who are required to report serious adverse events to the FDA.
The FDA previously told DailyMail.com about the figures: ‘While the FDA relies on the FAERS database as a drug safety monitoring tool after a product is approved and marketed, the submission of a report It does not mean that the information included therein has been medically confirmed. .
“The event may have been related to the underlying disease being treated, or may have been caused by the simultaneous taking of some other medication, or may have occurred for other reasons.”
They added: “Duplicate reporting and increased awareness of an event with a particular product may inflate the reported occurrence of an adverse event.”
Compared to 100 deaths related to weight loss drugs each year, 16,000 are attributed to NSAIDs every 12 months. — nonsteroidal anti-inflammatory drugs used to treat pain and inflammation, such as ibuprofen and aspirin.
The FDA previously warned that in rare cases Ozempic can cause an intestinal blockage, called ileus, because it slows the passage of food through the intestines.
This can cause them to break down and leak their contents into the body, which can lead to sepsis and multiple organ failure if not treated quickly.
Many patients taking the drug are also obese, which puts them at greater risk for serious side effects from any complications caused by the drug.
Ozempic and its competitors have risen to prominence in recent years, with a whopping 9 million prescriptions written for these drugs in the last three months of 2022 alone, the last available, which is a 300 percent increase in three years.
Eli Lilly’s Zepbound surpassed Wegovy in March, the data showed, with 6,000 more prescriptions issued in a month for a total of 77,590.
Doctors, consumers, manufacturers, family members, and others can report deaths to FAERS.
No medical documents are requested with the initial report, but those who submit it will be asked for detailed information about the adverse event.
FDA investigators constantly review and monitor reports for drug side effects that may not have been detected in clinical trials.
The agency previously used them to add the side effect ileus to the warning label, or a partial or complete obstruction of the small intestine.

