Farms in several states are quarantining cows for fear of a rapid spread of bird flu.
Health officials in 18 states have imposed restrictions on livestock imports from states where bird flu, also known as H5N1, has been confirmed in dairy cows.
Although officials say the risk to humans who eat beef is “very low,” some experts are concerned about a possible outbreak in humans, after a Texas dairy farmer became the second American to test positive for avian flu.
Last week, Dr. Darin Detwiler, former FDA and USDA food safety advisor, told DailyMail.com that Americans should avoid raw meat and liquid eggs while the outbreak in livestock was ongoing.
In humans, severe infections can lead to respiratory failure, brain inflammation, and multiple organ failure.
Last week, Maryland and North Carolina became the latest states to enact limits on cow movement, when North Carolina detected its first infection.
Experts fear that infections in cattle and other mammals could increase the risk of the virus adapting to spread among humans.
The list also includes Alabama, Arkansas, California, Florida, Hawaii, Nebraska and Tennessee, according to the American Veterinary Medical Association (AMVA).
States like New York have also issued warnings to residents to stay away from local wildlife, such as geese, hawks and falcons, which can also carry the deadly virus.
Starting April 2, the USDA said it will not issue federal quarantine orders or recommend state-level restrictions.
‘However, we strongly recommend minimizing livestock movement as much as possible, with particular attention to assessing risk and factoring this into movement decisions. “Do not move sick or exposed animals,” the agency said.
USDA Animal and Plant Health Inspection Service (APHIS) officials said that if livestock need to be moved, producers, veterinarians and health officials are encouraged to use “extreme diligence” to ensure that only healthy cattle are moved.
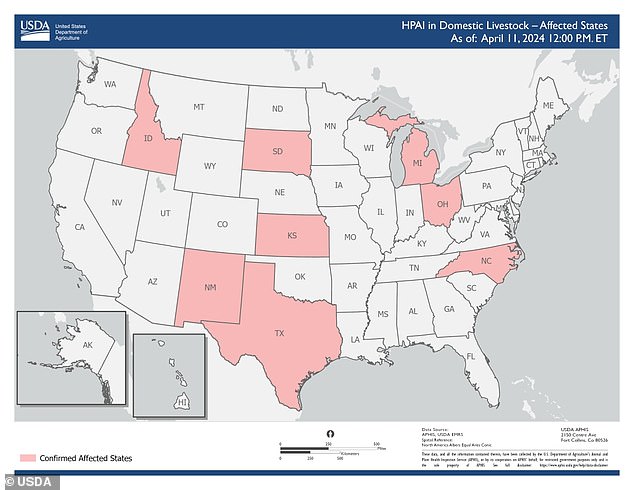
The map above shows states with cattle herds that have been diagnosed with bird flu.
Each state has different restrictions. For example, Maryland, the most recent state to establish limits, bans dairy cattle in states with confirmed bird flu detection.
Avian flu is a disease caused by infections with the influenza A virus, which tends to spread to waterfowl. It can then infect farm animals such as poultry and cattle.
So far, 28 farms in eight states have reported H5N1 infections in their cows, including 11 in Texas and six in New Mexico.
States such as Iowa, California and Minnesota have begun testing their animals for the virus. Infected cattle are described as “lethargic”, eating less feed and producing less milk.
But it’s unclear how the cows became infected, whether through exposure to infected droppings, bird carcasses or another route.
However, some researchers suggest that livestock are becoming ill after drinking contaminated water from birds migrating through the area.
Earlier this month, a Texas farmer became the second American to test positive for H5N1. The CDC says the patient had a “mild” infection with only one symptom – eye swelling – and has reported that he is in isolation and “recovering well.”
They are also being treated with the drug oseltamivir or Tamiflu and are not believed to have transmitted the virus to anyone else.
Texas has also reported 11 farm outbreaks, the most of any state.
There are still many unknowns surrounding the human case.
It could have been from direct contact with cows or from touching a surface contaminated with the virus and then touching their faces.
Although many mammals are being infected, experts say there is one species they are focusing on in particular: pigs.
These animals have the same receptors in their lungs as humans, meaning an outbreak among them could predict a similar episode in humans.
However, no infections are currently recorded in pigs.
According to the CDC, some humans infected with bird flu may not experience symptoms.
Those who do have reported flu-like symptoms, such as fever, cough, sore throat, body aches, headaches and fatigue.
Earlier this month, the CDC told state health officials to prepare for more human cases of bird flu, including “updated operational plans” in case more farmworkers test positive. However, the agency also emphasized that the risk to the public is low.
This came after CDC Director Dr. Mandy Cohen said the agency is taking the bird flu “very seriously.”
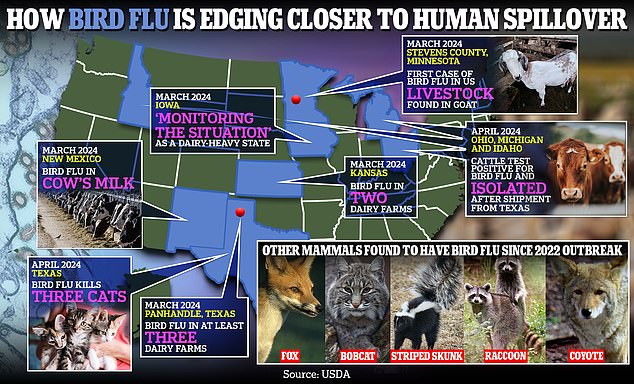
The above shows how bird flu is approaching human contagion in the US.
Dr. Darin Detwiler said that while the chance of getting bird flu from properly cooked food is low, “the severity is high” once someone gets sick.
“The risk increases with undercooked eggs or meat, just as the risks increase in a car accident if you don’t wear a seat belt.”
“However, when we look at the severity, the H5N1 strain has caused severe illness and death in infected humans.”
He noted that serious complications include respiratory failure, encephalitis (inflammation of the brain), acute respiratory distress syndrome (ARDS, which causes fluid to build up in the lung alveoli), and multiple organ failure.
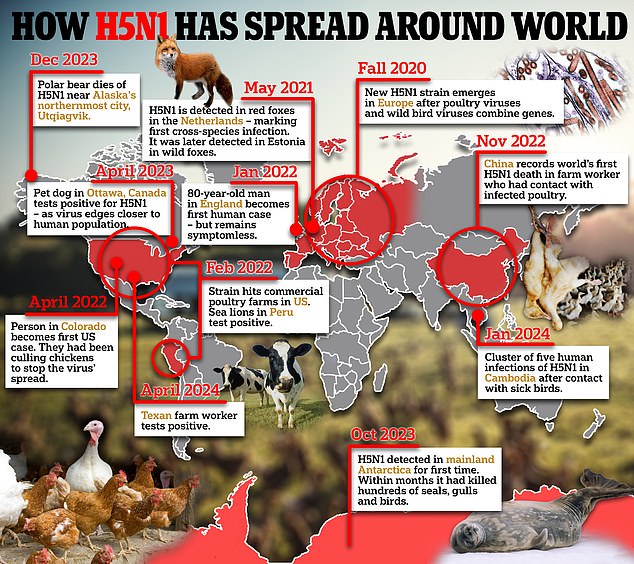
The World Health Organization estimates the mortality rate from H5N1 at 52 percent, based on the 462 deaths recorded since 2003 among the 887 people diagnosed with the virus.
Dr. Detwiler also warned against eating foods containing undercooked meat and eggs, such as rare steak, eggs Benedict, and even Caesar salad, to reduce the risk of transmission.

