A gene therapy once considered “the most expensive drug in the world” will be offered for free on the NHS, but experts insist it will save the health service money.
The drug, called Hemgenix, costs £2.6 million per patient, although only one dose is needed.
It is the only treatment of its kind for hemophilia B, a bleeding disorder in which the body does not make enough, or any, of a protein critical to clotting.
Coagulation is a vital biological mechanism that stops bleeding from wounds, meaning that people with this disorder can suffer serious and even life-threatening blood loss if they suffer an injury.
In addition, they are also at risk of what are called ‘spontaneous bleeds’ which occur without direct injury and are life-threatening if they occur in a vital organ.
The drug, called Hemgenix, costs £2.6m per patient but experts insist it could save the NHS money in the long run.
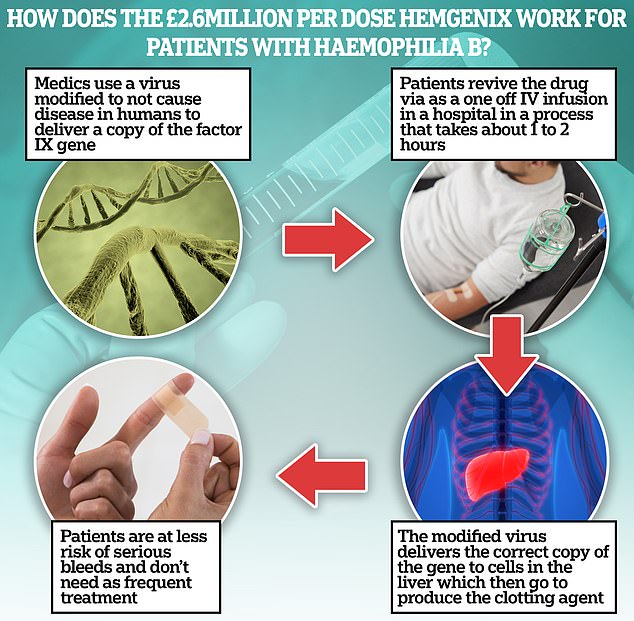
The Hemgenix patient’s defective gene, which is unable to produce clotting, is replaced with one that can, eliminating the need for frequent injections.
Patients with hemophilia B must receive frequent, lifelong injections of an artificial clotting agent called Factor IX to keep the risk of catastrophic injury to a minimum.
But with Hemgenix the patient’s defective gene, which is unable to produce clotting, is replaced with one that can, eliminating the need for these injections.
Studies on the gene therapy, administered via intravenous drip, have shown that the effects last at least three years, but it is hoped it could work even longer.
Although there are approximately 2,000 people with haemophilia B in the UK, it is estimated that only 260 are eligible for Hemgenix on the NHS.
This puts the total potential bill for the taxpayer at around £676 million.
However, MailOnline understands that the NHS is purchasing the drug at an undisclosed discount, so the actual cost is likely to be lower.
The UK’s National Institute for Health and Care Excellence (NICE), which decides which medicines should be available on the NHS and to whom, recommended that the medicine be made available in the health service on a “managed access” basis. .
This means it will only be offered to patients with “moderate or severe hemophilia B” whose doctor believes they would benefit.
It will also be subject to an ongoing review of its profitability. This could mean it will one day be rolled out to more patients.
One patient already benefiting is Elliott Collins from Essex.
The 34-year-old man suffers from severe hemophilia B and participated in gene therapy trials five years ago.
On the Today show, Collins said he was effectively no longer a haemophiliac thanks to treatment.
“Initially my coagulation levels were below zero and now they hover around 60, and anything 50 or higher technically you’re not a hemophiliac,” he said.
“Not long after getting the treatment, I remember walking by and hitting my knee on a closet… and at first I thought, ‘Ooh, I hit it hard enough that it’s swollen up quite a bit and I’m going to need treatment.’ ‘.
‘And then 20 minutes went by and nothing, maybe a small mark, whereas before that would surely have swelled up like a peach and I would have had to treat myself.
“This is real, it’s working and I can relax a little.”
Professor Amit Nathwani, a haemophilia expert at University College London who helped develop the gene therapy, said that while on paper the cost per patient is staggering, using the drug could in fact save the NHS money.
“It’s a lot of money, but if you look at the lifetime cost of gene therapy with factor replacement concentrate, it’s over £8 million per patient,” he said.
“Therefore, a single administration of gene therapy leading to long-term, potentially lifelong protection could be a bargain, it could represent a substantial saving for the NHS.”
He added that this does not take into account other potential savings for the health service, such as reducing the need for life-saving operations for hemophilia B patients.
“Even with regular injections of factor IX, patients continue to bleed and have life-threatening hemorrhages; with gene therapy, the data show that these spontaneous hemorrhages are almost completely eliminated,” he said.
Clive Smith, chairman of the charity Haemophilia Society, welcomed the news of the approval, calling it a “huge step forward” for people living with haemophilia B.
“It has the potential to significantly improve the quality of life of those who are eligible for such treatment,” he said.
“Gene therapy offers people the opportunity to effectively eliminate painful bleeds, thereby improving joint health and allowing people to live full lives, unrestricted by frequent infusions and trips to the hospital.”
NHS England’s most senior medical professor, Sir Stephen Powis, also praised the news.
“This promising drug is the latest in a series of pioneering gene therapies secured for NHS patients at an affordable price and becomes the first drug available in our Innovative Medicines Fund to provide early access to patients while more data on its long term “benefits,” he said.
NHS England added that haemophilia B patients interested in the drug can discuss Hemgenix eligibility with the healthcare professional responsible for their care.
Hemgenix is manufactured by Philadelphia-based pharmaceutical company CSL Behring, and Eduardo Cabas, managing director of its UK and Ireland operations, welcomed NICE’s decision.
“CSL Behring is proud to be a global leader in biotechnology innovation and we remain clearly committed to improving the lives of people living with rare genetic bleeding disorders, as demonstrated by this milestone for eligible haemophilia B patients in the UK and the NHS,” he said.
Hemgenix was initially described as the “world’s most expensive drug” when it originally appeared on the scene in 2022.
However, other medications have since eclipsed it in terms of overall cost.


