An experimental weight-loss drug could burn fat up to five times faster than the drug behind Ozempic and Wegovy, trial results suggest.
Overweight and obese patients who received the new weekly injection lost up to 32 pounds, or 14.7 percent of their body weight, on average in just 13 weeks.
For comparison, clinical trials of the drug at Ozempic and Wegovy found that patients only lost that amount of weight in 68 weeks, or five times as long.
The California-based biotechnology company that makes the drug, Viking Pharmaceuticals, saw its stock double in value after the results were revealed, giving it a stock market value of $8.5 billion. The company’s stock price has more than quadrupled so far this year.
Doses of the experimental drug were administered up to 15 milligrams (mg) per week, much higher than the regimen of other weight-loss drugs (file image)
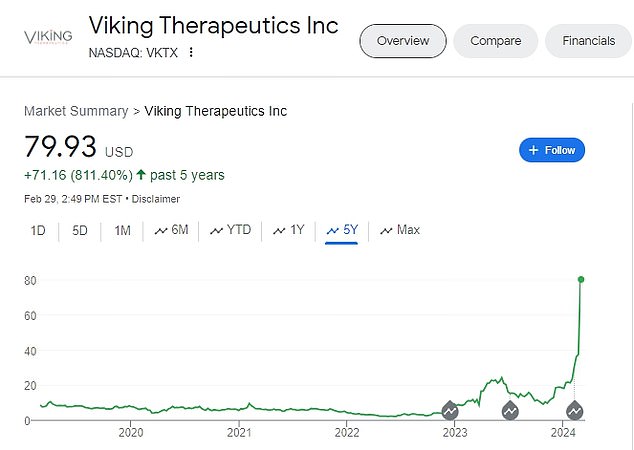

Viking’s share price has more than doubled since the announcement. Above is the company’s share price value over the past five years.
Viking reported that patients in its trial had adverse events such as nausea and vomiting, but said the cases were mild or moderate.
This was similar to the Ozempic and Wegovy trials, where patients also reported these side effects.
One patient in the trial of the drug, called VK2735, had a severe case of dehydration.
Tirzepatide from Ozempic, Wegovy, and Eli Lilly works by stimulating natural hormones that regulate blood sugar and suppress appetite.
Viking’s drug acts on two receptors like tirzepatide, but the phase two trial suggested its injection was even more effective than the latter.
However, the Vikings will still have to go through Phase Three testing. – to evaluate its safety and effectiveness in a larger population – before confirming that success.
After phase three, it will also be eligible for Food and Drug Administration (FDA) approval. The company said it is currently in talks with the agency.
The price of the new drug has not been revealed, but Ozempic costs more than $900 a month out of pocket.
Viking still needs to find a distribution partner to mass produce the drug.
The development comes as pharmaceutical companies try to cash in on the U.S. weight-loss drug market, which is expected to grow to $44 billion by 2030 from less than $100 million in 2020.
More than nine million prescriptions for drugs, including Ozempic, Wegovy and Mounjaro, were issued in the last three months of 2022, and companies rushed to expand supply to meet demand.
Viking Pharmaceuticals says its drug works by mimicking the hormone glucage-like peptide-1 (GLP-1), the same one in Ozempic that makes patients feel fuller longer.
But the drug also mimics glucose-dependent insulinotropic polypeptide (GIP), which can help slow the movement of food through the intestine, ensuring someone feels fuller longer.
In the Viking study, scientists recruited 176 overweight or obese adults with at least one underlying comorbidity, such as type 2 diabetes.
They weighed 225 pounds on average at the start of the study.
Initially, participants were divided into five groups of approximately 35 adults each, one of which received placebo while the other four were randomly assigned to doses of VK2735 ranging from 2.5 to 15 mg.
The scientists then monitored their weight for 13 weeks while they received weekly injections of the medications, as well as any reported side effects.
The trial was double-blind, and neither the scientists nor the participants were told who was receiving the new drug and at what dose.
The results showed that 88 percent of patients in the group receiving the highest dose achieved weight loss of at least 10 percent of their starting body weight.
People in this group lost an average of 32.2 pounds during the study period, which is equivalent to 2.5 pounds per week.
For comparison, the least amount of weight loss was recorded in the group that received 2.5 mg per week: 20 pounds more than the study on average or 1.5 pounds per week on average.
In the placebo group, only four percent had weight loss at this level during the same period.
Patients in the higher dose group were also more likely to report side effects: 40 percent said they were nauseated and 30 percent said they were vomiting.
In comparison, those who received the lowest doses were less likely to experience side effects: Nine percent said they suffered from vomiting.
It was unclear what proportion of the weight lost was fat compared to muscle, and the problem in previous trials was that patients had mainly lost muscle, leading them to regain weight quickly when they returned to their previous diet.
Revealing the results, Dr. Brian Lian, CEO of Viking, said: “We are excited to report the topline results from this important phase two study.
A total of 18 of 176 patients (or 10 percent) also stopped treatment while in the study.
“VK2735 continues to demonstrate a promising efficacy and tolerability profile after 13 weeks of repeat dosing in obese subjects.”
He added: ‘In particular, significant weight loss compared to placebo was observed early at all doses evaluated in the VENTURE study (phase two trial), and continued throughout the treatment period in all groups. of treatment.
‘No evidence of a plateau was observed at week 13 for any dose of VK2735, suggesting that greater weight loss could be achieved with prolonged dosing periods.
“We look forward to moving this important therapy into further clinical development later this year.”
The company is also working on an oral formulation of its drug, similar to the one being developed by Novo Nordisk, the maker of Ozempic.

