<!–
<!–
<!– <!–
<!–
<!–
<!–
Up to 10,000 people in the UK could have rare genetic quirks that make them up to six times more likely to become obese, a study suggests.
Obesity affects around a quarter of all adults in the UK and increases the risk of diseases such as type 2 diabetes, stroke and coronary heart disease.
But the genetic reasons why some people are more likely to gain weight are not well understood.
Now, researchers have discovered genetic variants in two genes that have some of the largest impacts on obesity risk to date.
Your browser does not support iframes.
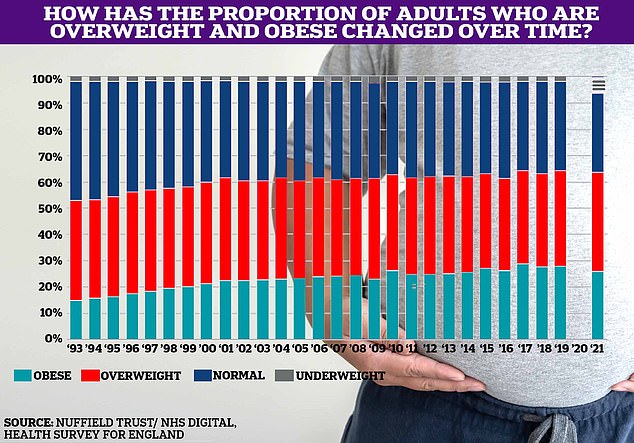
The proportion of Britons who are overweight or obese has slowly increased over time, reaching two-thirds in 2021, the latest data available. No data was recorded for 2020, the year of the Covid pandemic.
The team, based at the University of Cambridge, analyzed the genes of more than 500,000 people in the UK to look for rare variants responsible for obesity.
They found that genetic variants in the BSN gene, also known as bassoon, can increase the risk of obesity by up to six-fold.
The quirk was also linked to an increased risk of nonalcoholic fatty liver disease and type 2 diabetes.
Bassoon gene variants were found to affect one in 6,500 adults, so could affect around 10,000 people in the UK.
A rare variant of a gene called APBA1 was also found to increase the risk of obesity.
The researchers said these are some of the first obesity-related genes identified whose increased risk does not affect children, only adults.
They suggest that the Fagot and APBA1 genes play a role in transmitting signals between brain cells, which could begin to affect appetite control as a person ages.
Study author Professor John Perry said: “These findings represent another example of the power of large-scale genetic studies of human populations to improve our understanding of the biological basis of disease.”
“The genetic variants we identified in BSN confer some of the largest effects on obesity, type 2 diabetes, and fatty liver disease observed to date and highlight a new biological mechanism that regulates appetite control.”
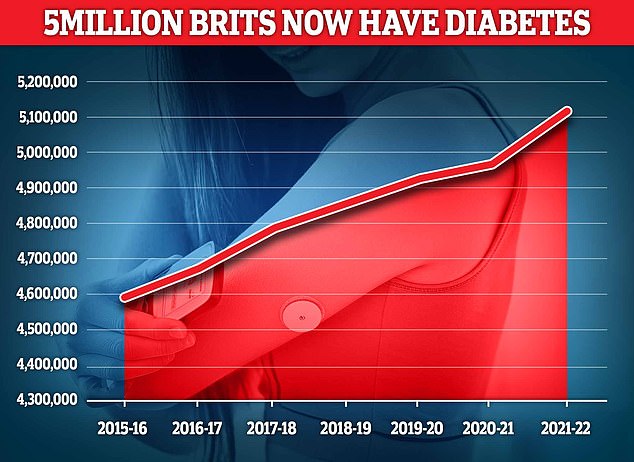

Almost 4.3 million people were living with diabetes in 2021/22, according to the latest UK figures. And another 850,000 people have diabetes and are completely unaware of it, which is worrying because untreated type 2 diabetes can lead to complications such as heart disease and stroke.
The researchers said that understanding the neural biology of obesity could present more potential drug targets to treat the condition in the future.
Professor Giles Yeo, who also worked on the study, said: “We have identified two genes with variants that have the most profound impact on population-level obesity risk that we have ever seen, but perhaps more importantly, the variation in bassoon is related to adult-onset obesity and not childhood obesity.
“These findings therefore offer us a new appreciation of the relationship between genetics, neurodevelopment and obesity.”
As part of the study, researchers worked with biopharmaceutical company AstraZeneca to test their findings in cohorts of patients from other countries.
This is important, they said, because it shows that they can apply their findings beyond individuals of European ancestry.
Dr Slavé Petrovsky, Vice President of the AstraZeneca Genomic Research Centre, said: “Rigorous, large-scale studies like this are accelerating the pace at which we discover new insights into the biology of human diseases.
“By collaborating between academia and industry, leveraging global data sets for validation, and incorporating a genomic approach to medicine more broadly, we will continue to improve our understanding of diseases, for the benefit of patients.”
