Scientists believe they are on the verge of creating the “holy grail” of weight-loss drugs that reduce appetite without the nauseating side effects.
Early studies show that a drug can effectively “activate” certain cells in the nervous system that play an important role in maintaining energy balance and sugar control.
It could eventually rival companies like Wegovy and Mounjaro, two coups that have already transformed the future of weight loss and helped millions of people around the world lose weight.
While these two are very effective, they can cause debilitating side effects like nausea and nausea, meaning some people can’t continue using them.
Now, new research shows that activating certain cells within the body’s nervous system, called neurokinin 2 receptors (NK2R), increased calorie burning and reduced appetite without any signs of nausea.
This technique was also found to reduce appetite without loss of muscle mass, another possible side effect of current treatments.
Researchers at the University of Copenhagen tested the effect of activating NK2Rs, which they believed plays a role in maintaining energy balance and glucose control.
Trials first conducted in mice found that activating the receptor safely increases calorie burning and also reduces appetite without any signs of nausea.
It could eventually rival companies like Wegovy and Mounjaro, two coups that have already transformed the future of weight loss and helped millions of people around the world lose weight.
This, they say, is especially important given that our bodies seem to burn fewer calories at rest than they did a few decades ago.
Other studies in primates with type 2 diabetes and obesity showed that NK2R activation reduced body weight and reversed their diabetes by increasing insulin sensitivity and lowering blood sugar, triglycerides, and cholesterol.
The scientists said the findings, published in the journal Nature, represent “a major step forward” in the development of new drug therapies for those suffering from type 2 diabetes and obesity.
Zach Gerhart-Hines, associate professor of metabolic research, said, “While GLP-1-based therapies have revolutionized the care of patients with obesity and type 2 diabetes, safely harnessing energy expenditure and controlling appetite without nausea They remain two holy grails in this field.
“By addressing these needs, we believe our discovery will advance current approaches to make more tolerable and effective treatments accessible to millions more people.”
Earlier this month, the UK’s medicines watchdog received reports of ten deaths linked to the use of weight-loss injections, it revealed.
There have also been 7,228 reports of nausea, vomiting and diarrhea associated with companies such as Wegovy and Ozempic.
Of those, 68 patients were admitted to hospital, the Medicines and Healthcare products Regulatory Agency (MHRA) said.
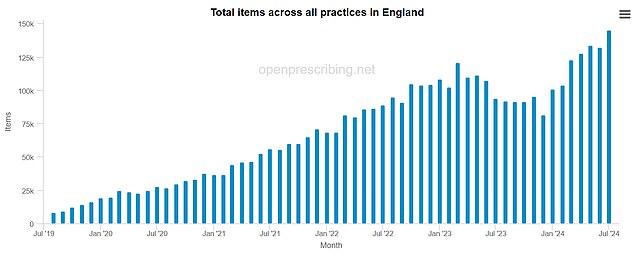
The NHS-backed OpenPrescribing data source shows soaring prescriptions for semaglutide, the drug in Ozempic and Wegovy.
The figures are based on users or healthcare workers reporting adverse reactions to the drugs, known as glucagon-like peptide-1 receptor agonists (GLP-1RA), to the regulator.
A reported death or adverse reaction does not necessarily mean it was caused by the drug, just that someone suspected it may have been that way.
Underlying or concurrent diseases and other medications that patients may have taken at the time of their death may be responsible and such events may also be coincidental, he told the trade journal Chemist and Druggist.
Last week, the MHRA urged healthcare professionals to “report cases of misuse” and “inform patients about common and serious side effects associated with GLP-1RAs”.
At the time, it said it knew of 46 hospitalizations as of Aug. 16, suggesting there have been 22 additional reports in two months, representing a 48 percent increase.
The alert warned healthcare professionals to “be aware that there have been reports of possible misuse of GLP-1RA for off-label indications, such as cosmetic weight loss.”
The regulator said that “healthcare professionals should… be alert for signs of misuse of these medicines in their patients, warn them that they are at risk of side effects and report any adverse reactions.”
It added that patients should also be warned about the risk of counterfeit GLP-1RA weight loss medicines if not prescribed by a registered healthcare professional and note that some counterfeit medicines have been found to contain insulin.