Boeing’s chief financial officer said the company would use more cash in the first quarter than expected to try to combat the aircraft maker’s deepening crisis.
Chief Financial Officer Brian West told a Bank of America conference that the company would spend between $4 billion and $4.5 billion.
West disputed that this figure was “higher than we initially forecast in January” and said it would produce less than the maximum of 38,737 planes allowed each month.
This is due to a combination of lower deliveries, lower production volumes in its trading division as well as some pressure on working capital.
Quality at Boeing and its supplier Spirit AeroSystems is under scrutiny following a January incident in which a door jam caused a 737 MAX 9 plane to blow up in mid-flight.

Chief Financial Officer Brian West, seen here with his wife Sheri West, told a Bank of America conference that the company would spend between $4 billion and $4.5 billion.


In early January, an unused emergency exit door blew up a brand-new Boeing 737 Max shortly after takeoff from Portland International, sparking a still-ongoing Justice Department investigation.
West said: “We’re going to deliberately slow down to get it right. It was we who made the decision to constrain prices on the 737 program. And we will feel the impact over the coming months.
It will also take longer for Boeing to reach its 2022 target of around $10 billion in annual cash flow by 2025 or 2026.
He added: “It’s going to take us longer than expected to get there. But we believe the steps we are taking now position us better for the long term.
West said margins in the commercial aircraft business would be “more like 20% negative” in the first quarter, in part because of customer compensation for delivery delays.
They will improve throughout the year but will remain negative overall in 2024, he added.
Quality at Boeing and its supplier Spirit AeroSystems is also under scrutiny following an incident in January in which a door plug exploded from a 737 MAX 9 plane in mid-flight.
The door panel that exploded from the 737 MAX 9 jet appeared to be missing four key bolts, according to a preliminary report from U.S. investigators.
“For years we prioritized moving the plane through the factory over getting it done right, and that needs to change,” West said.
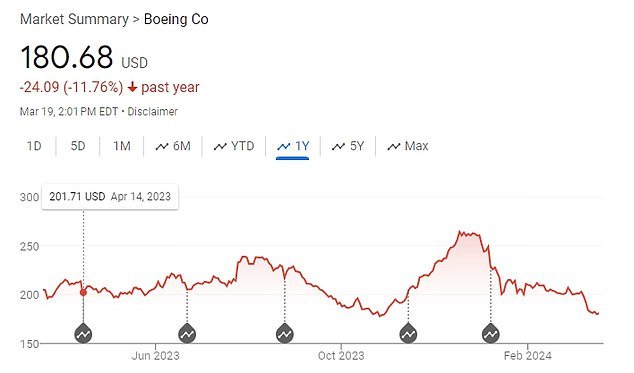

Boeing’s stock prices have fallen over the past year following several incidents.
In the January incident, an unused emergency exit door caused a brand-new Boeing 737 Max to explode shortly after takeoff from Portland International, sparking a still-ongoing DOJ investigation.
Last week, around 50 people were treated by first responders after a Boeing 787 Dreamliner flying between Australia and New Zealand experienced a “technical event” that caused “strong movement” shaking passengers in their seats.
The company on Friday asked airlines to begin inspecting pilot seat switches after a report indicated that accidental movement of the cockpit seat caused the fall.
Shortly before the incident over the Indian Ocean, Boeing said it believed the technical failure involving the door stemmed from something that happened during production, where required documents detailing the removal of a faulty key part had never been created.
Also Friday, the company said it is “committed to continuing to cooperate fully and transparently with the National Transportation Safety Board’s investigation,” which, more than three months later, continues.
This all comes after Boeing whistleblower John Barnett was found dead in his truck in a South Carolina hotel parking lot, seven years after retiring.


Barnett had alleged that second-rate parts were literally being taken out of the trash, before being fitted to planes built to avoid delays.


The 62-year-old was found in his truck in a hotel parking lot in South Carolina, seven years after retiring from a 32-year career with Boeing.
Barnett’s death came during a whistleblower lawsuit, in which he alleged that under-pressure workers were deliberately installing substandard parts on planes on the assembly line.
Barnett had alleged that second-rate parts were literally being taken out of trash, before being fitted to planes built to avoid delays.
A 2017 review by the FAA confirmed some of its concerns, forcing Boeing to take action.
He had just filed a deposition with Boeing lawyers in the case the week before his death, said his lawyer, Brian Knowles.
The Charleston County coroner confirmed last week that the longtime Boeing employee died while in town for interviews related to the case.
Boeing also responded to the former worker’s death in its own statement as the news spread, saying it was “saddened by the passing of Mr. Barnett.”
The statement did not address any aspect of the case, but ultimately added: “Our hearts go out to his family and friends.”


A Boeing plane was recently forced to land due to hydraulic fluid leaking from its landing gear area. Under investigation, the technical failure also occurred mid-flight on a United flight.
Barnett’s job for 32 years was to oversee the company’s aircraft production standards – standards he said were not met during his four years at the new Charleston factory, from 2010 to 2014 .
“The new management didn’t understand the processes,” Barnett told the Corporate Crime Reporter in a 2019 interview about how the brass allegedly cut corners to get their then-state-of-the-art 7878s out on time.
“They brought them in from other parts of the business,” he continued, two years after retiring in 20017.
“This whole team went down,” he continued. “They were on the military side. My impression was that their mindset was: we’re going to do it the way we want to do it.
“Their motto at the time was: We’re in Charleston and we can do whatever we want.”
Barnett claimed to have alerted plant superiors of his concerns, but no action was ever taken. Boeing has denied this, as well as its claims.
A 2017 review by the Federal Aviation Administration (FAA) later dispelled some of Barnett’s qualms, including revealing that at least 53 “non-conforming” parts – as they put it – were misplaced and considered lost.
The company is now under criminal investigation for the Max plane door incident last January.
