Russia could be using a top-secret electronic weapon to disrupt the GPS systems of planes and ships, experts have warned, as thousands of British holiday flights are believed to have fallen victim to “extremely dangerous” signal jamming attacks.
Military chiefs have warned in recent months that a system known as Tobol, based in the Russian enclave of Kaliningrad, could be jamming signals from planes and ships, causing them to “malfunction.”
Last night it emerged that airliners appear to have faced GPS jamming and spoofing, which interferes with wireless communication systems and uses false signals to trick pilots into believing the plane is in a different location than it actually is.
The European Aviation Safety Agency warned in January that authorities had seen a “sharp increase” in jamming and spoofing “attacks,” but did not say who was behind them.
Shortly afterwards, an RAF plane carrying Defense Secretary Grant Shapps had its signal jammed while flying near Kaliningrad, and insiders blamed Russia for what they called a “wildly irresponsible” attack.
The suspected electronic weapon causing these disturbances is likely based on Russia’s military site in Kaliningrad (pictured), located between Lithuania and Poland, according to Western intelligence findings.
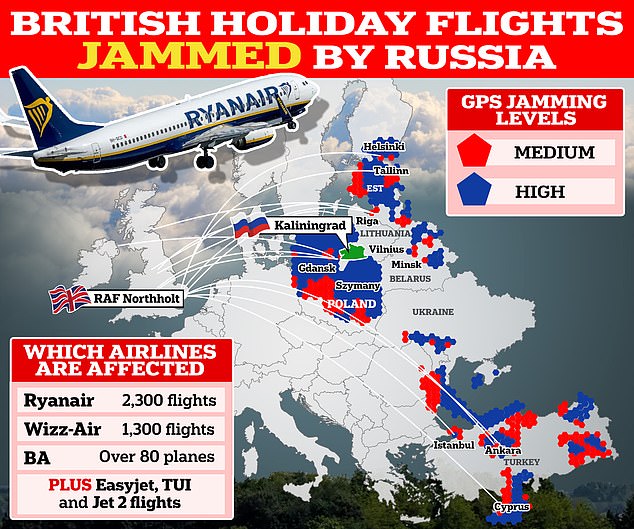
Aircraft logs have revealed jamming hotspots in the Baltic region, the Black Sea and the eastern Mediterranean, with a map showing where attacks have allegedly occurred.

Vladimir Putin’s forces have been accused of using a secret weapon to jam GPS signals.
A defense source reportedly said at the time: “While the RAF is well prepared to deal with this, it still poses an unnecessary risk to civilian aircraft and could potentially put people’s lives at risk.”
The predicted threat to civil aircraft appears to have been confirmed, with Sun reported yesterday that 46,000 flights reported satellite navigation (SATNAV) problems in the Baltic region between August and March.
These included 2,309 Ryanair flights, 1,368 Wizz Air flights, 82 British Airways flights and four EasyJet flights.
The electronic weapon suspected of causing disruption to aircraft in the area is likely based on the Russian military site in Kaliningrad, a Russian territory between Lithuania and Poland, according to Western intelligence findings.
It is reported to be a large satellite dish, although images circulating on social media claiming to show the device have not been verified. Aerial shots show the suspicious site.
According to Estonia’s military chief, there has been a disruption in GPS guidance of air and sea traffic in Finland, the Baltic countries and Poland in recent months.
“What we have seen is a malfunction of GPS for ships and air traffic,” General Martin Harem, commander of the Estonian Defense Forces, told the Telegraph.
‘And we really don’t know if [Russia] They want to achieve something or just practice and test their equipment.

The Kaliningrad fixed jamming system is said to be one of ten such facilities in Russia. Pictured: A photograph believed to show the site.

It is reported to be a large satellite dish, although images circulating on social media claiming to show the device have not been verified. An aerial shot shows the suspicious site.
Now, aircraft logs have reportedly revealed jamming hotspots in the Baltic region, the Black Sea and the eastern Mediterranean, with a map showing where attacks allegedly occurred.
Tobol likely works by transmitting a signal on the same frequency as the plane or ships’ satellites, known as downlink jamming, preventing them from receiving the legitimate signal. Washington Post reports citing an expert on the program.
While the fixed jamming system in Kaliningrad is said to be one of ten such facilities throughout Russia, its strategic position near Ukraine, along with another site in Crimea and one outside Moscow, makes it suitable for both operations “offensive” as well as “defensive.” operations.
Brian Weeden, director of program planning at the Secure World Foundation, said that if Tobol is capable of analyzing signals and transmitting passwords, “you can probably use those same capabilities to offensively interfere with someone else’s satellite.”
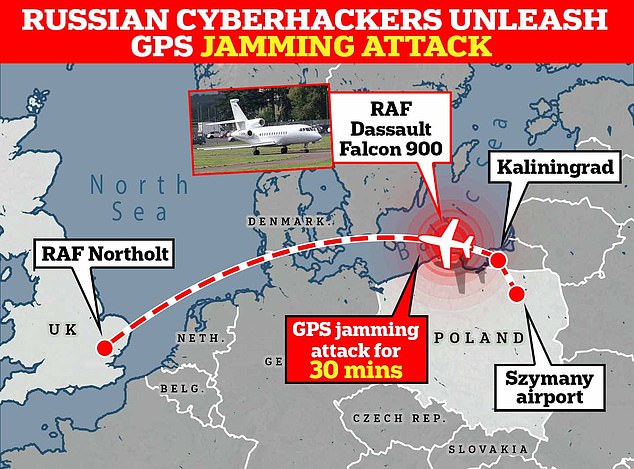
This is the flight path Shapps’ plane took before being hit by a Russian cyberattack outside Kaliningrad.

An RAF plane carrying Defense Secretary Grant Shapps had its signal jammed while flying near Kaliningrad.
Dr Jack Watling, a war expert at the Royal United Services Institute think tank, told The Sun that Russia has “long used GPS jamming as a tool of harassment” and that the country is “projecting it beyond NATO’s borders.
“Wherever there is a large Russian garrison you see GPS denial and there is one in Kaliningrad,” he said. “They just have those things on because there are standing orders.”
An electronic warfare expert said the satellite dish could be used to alter GPS technology in several directions, which could protect Kaliningrad from possible incoming missiles.
RUSI’s Dr Thomas Withington told the Telegraph earlier this year that the device was likely used as a defensive weapon due to Putin’s fears about GPS-enabled weapons available to other countries.


The fixed jamming system is called Tobol and is said to be one of ten installations in use throughout Russia. It reportedly looks like a large satellite dish, but images circulating on social media claiming to show the device could not be verified.
Causing a GPS disruption on these generally high-precision missiles could cause them to miss their target.
However, if the weapon is used against civilian rather than military targets, it can wreak havoc on commercial airliners.
There have recently been reports of aircraft suddenly leaving tracking sites, probably due to an outage in their GPS.
Experts warned that if the same thing happens to ships, they could crash because they cannot be seen in navigation systems.
While ships have other means of navigation available, it would be a cause for concern if these systems failed, Dr Withington explained.
Any lasting GPS outage could cause logistics chaos as delivery drivers rely on it to reach their destinations.
Gen Harem said: ‘Whatever they do [Russia] “One of the goals we do here is to degrade our stability, our self-confidence, our trust in the West, our unity and our cohesion.”
Just hours after the electronic warfare attack on Shapps’ plane, the United Kingdom, the United States and new NATO member Sweden sent electronic surveillance aircraft to the Baltics.
The threat posed by Russian electromagnetic and electronic warfare is not limited to the airspace surrounding Kaliningrad.
The Kremlin has placed similar jamming systems in Syria in an attempt to jam unprotected aircraft and throughout Russia.

