Experts have mocked Tucker Carlson after suggesting Darwin’s theory of evolution has been discredited.
The host made the comments on The Joe Rogan Experience podcast, where he also stated that UFOs are piloted by “spiritual entities” that have hidden headquarters on Earth.
“There is no evidence of evolution,” Carlson said.
‘In fact, I think we have abandoned the theory of evolution as articulated by Darwin. It’s something that’s not true.’
Rogan pressed the issue and asked the announcer to explain his opinion, to which he stated that “there is no evidence that people evolved smoothly from a single-celled amoeba.”
Tucker Carlson argued that the theory of evolution has fallen out of favor. Scientists and even many Christians disagree.

Charles Darwin’s theory of evolution has been refined over time by modern scientists, but the central idea remains the same: species change over time to adapt to their conditions.
The conversation about evolution began with a casual comment from Rogan: “If evolution is real,” at which point Carlson interrupted to ask, “Is it real?”
“I don’t know,” Rogan responded, starting the discussion. But he is visible. It can be measured in certain animals.’
During his famous voyage to the Galapagos Islands in the 1830s, Darwin measured evolution in certain animals and discovered that mockingbirds were different from island to island.
Similarly, the finches he collected on different islands during the trip were so different from each other that they were clearly different species.
They probably started out as the same species of bird, Darwin concluded, but something changed when they migrated to new environments in the Galapagos.
As they adapted to their new environments, each new species developed physical characteristics that suited their survival.
Finches had different beaks depending on their habits: insect eaters had narrow beaks for digging into cracks, while nut eaters had thick beaks for cracking shells.
Scientists still agree that Darwin was right on this point.
“You can measure adaptation, but there is no evidence for evolution; in fact, I think we have abandoned the idea of evolution,” Carlson said. “The theory of evolution as articulated by Darwin is kind of false, right?”
‘In what sense?’ —Rogan asked.
“Well, in the most basic sense, the idea that all life arose from a single-celled organism and over time there would be a fossil record of that, and there isn’t,” Carlson responded.
Contrary to this point, Rogan noted that there is evidence in the fossil record of species in transition that have adapted to their environments.
An excellent example of this is Archeopteryx, a bird-like dinosaur that provided some of the first evidence of transitional species between groups known to science, in this case, between dinosaurs and modern birds.
Archeopteryx has a long tail and small teeth, like a dinosaur, but feathers and flight wings like a bird.

Charles Darwin’s finches have become a famous example of evolution. They probably started out as the same bird, but as they adapted to their new environments, each new species developed physical characteristics that suited their survival.

Transitional species like Archeopteryx provide strong evidence supporting the theory of evolution: This bird-like dinosaur had teeth and a tail like a dinosaur, but flight feathers and wings like a bird. Tucker Carlson dismissed transitional species as evidence of “adaptation,” not evolution: a false distinction.
It also had a bone called the furcula, which is a fused clavicle bone.
Only birds and dinosaurs had this feature, strongly suggesting that the extinct species was related to both.
Carlson, however, dismissed the “adaptation” examples, saying they do not prove that people evolved from single-celled organisms.
«There is no evidence (none, none) that people evolved smoothly from a single-celled amoeba. No, there is not. There is no chain of that in the fossil record,” Carlson argued.
And that’s why you don’t really hear people: you hear them referring to evolution, because the theory of adaptation is clearly and obviously true. But Darwin’s theory is totally unfounded; That’s why it’s still a theory, almost 200 years later.
Rogan didn’t press Carlson on the matter, except to ask him what his theories are.
“God clearly created people and animals,” Carlson responded. He has made no public statements about his faith, but Carlson tends to speak in support of Christian ideas.
In this case he cited the biblical book of Genesis, in which God created humans and animals.

The fused clavicle of Archeopteryx provided strong evidence of its shared relationship with dinosaurs and modern birds.

Tucker Carlson argued that humans were created, that we did not evolve from lower life forms like single-celled amoebas.
The way Carlson phrases the question, there is no room for argument.
But his simple answer actually contradicts how varied Christian opinions are when it comes to evolution.
According to a 2019 Pew Research Survey Of Americans, between 32 and 62 percent of white evangelical Protestants said they believed humans evolved over time, rather than always existing in their current form.
Among Catholic respondents, the numbers were even higher: Between 71 and 87 percent agreed that humans evolved over time.
Famed biologist EO Wilson has pointed out that fringe opponents of evolution cite Darwin to make it appear that evolution is an ideology that can be traced back to a single man, rather than a well-supported theory.
“It’s a rhetorical device to make evolution seem like some kind of faith, like ‘Maoism,'” he said. “Scientists don’t call it ‘Darwinism.'”
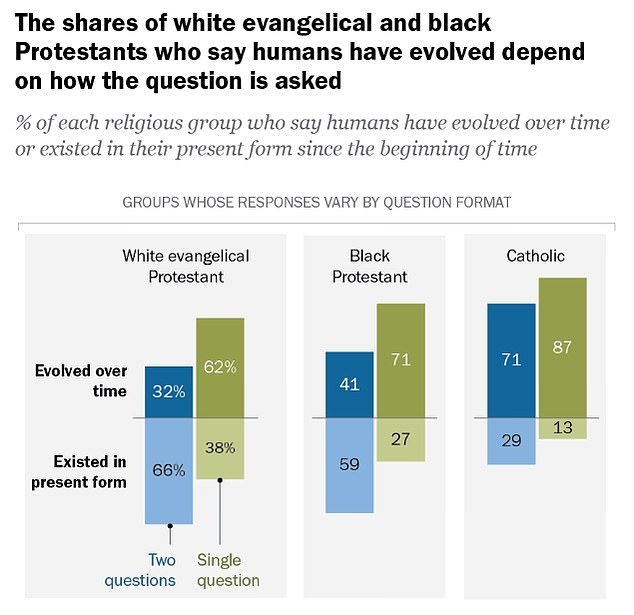
Many Christians in the U.S. actually believe in evolution, according to a 2019 Pew Research survey. However, people’s answers depend on how the question is asked.
And, in truth, Darwin did not come up with the theory of evolution. Rather, he refined the ideas of the scientists who came before him to develop his idea of ”survival of the fittest.”
Carlson is not the first person to attribute the theory of evolution solely to Darwin as a way of implying that it is on equal footing with his own ideas about human origins.
The announcer was quickly reprimanded for his comments by scientists, who correctly pointed out that Carlson misrepresented what a scientific theory is and that his comments ignored the mountains of evidence supporting the theory of evolution.
The commentator’s critics even included some of his frequent boosters, such as billionaire Tesla CEO Elon Musk.
Scientists have refined their explanations since Charles Darwin proposed the theory in the 19th century, but the central idea is the same, and evolution remains the agreed upon scientific explanation for how living things came to be the way they are.
“Tucker Carlson does not understand that in everyday speech, ‘theory’ refers to a hunch or a speculation, but in science it refers to a comprehensive explanation of nature,” wrote Clinical psychologist Jonathan Stea on X.
‘Many scientific theories are so well established that new evidence is unlikely to change them. For example: evolution,’ he said.
Musk responded to several such posts about X criticizing Carlson, expressing his agreement with them.
“I don’t agree with his views, but sometimes he makes good comments,” he said. wrote.
No fossil records have been found showing each stage of the evolutionary chain, but many experts say this is because it is very difficult to trace microbial life.
But DNA and RNA are so abundant in living things on Earth, another sign that we all evolved from a single thing.

