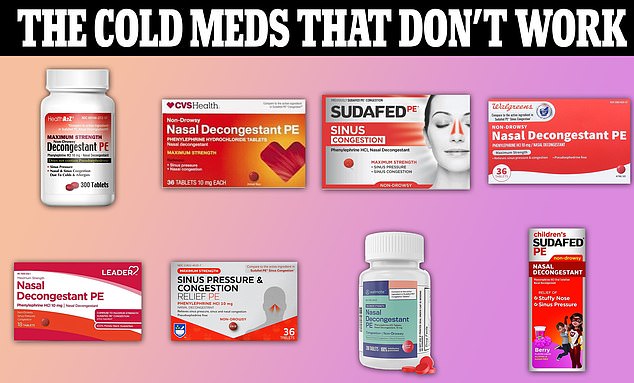
Thousands of cold and flu products could be on the chopping block after the FDA proposed removing medications containing phenylephrine from store shelves.
The agency this week proposed suspending the use of phenylephrine, an ingredient in common drugs like Sudafed, in over-the-counter products because it is “not effective.”
While sold under recognizable brand names such as Sudafed, the drug is also available under less expensive store brands, including pharmaceutical giants CVS, Walgreens and Rite Aide.
And when looking for a cold or flu remedy, consumers should pay special attention to the “active ingredients” label, which lists the medications that work in each product.
Any medication that includes only phenylephrine should be omitted, according to the FDA’s findings.
Some popular options include CVS Health’s Non-Drowsy Nasal Decongestant and Rite Aid’s Maximum Sinus Congestion and Pressure Relief.
Both products claim to temporarily relieve sinus pressure and nasal and sinus congestion due to allergies or the common cold.
Shortly after the FDA determined the ingredient was ineffective, CVS began recalling products that only contained phenylephrine.
Other well-known products include Sudafed children’s nasal decongestant and sinus congestion; and Walgreen’s Non-Drowsy Nasal Decongestant.
Similarly, these claim to provide relief from nasal congestion, sinus pressure, and nasal congestion.
But if phenylephrine is listed along with other active ingredients, the product may still be effective in treating other cold and flu symptoms.
Phenylephrine is frequently used in combination with diphenhydramine in the allergy medication Benadryl.
Diphenhydramine is an antihistamine that can treat allergy symptoms such as itching, congestion, watery eyes, sneezing, and runny nose.
Phenylephrine is also commonly used along with acetaminophen, the generic name for the pain reliever and fever reducer Tylenol.
The two drugs are used in combination in cold medicines to help treat congestion and sinus or headache pain, as well as relieve fever.
Some of these products include Vicks and Dayquil cold and flu therapies.
Finally, dextromethorphan, a cough suppressant, is often listed as an active ingredient along with phenylephrine.
Customers will find this combination in many Mucinex products seeking to decongest, relieve discomfort, and stop chronic coughs.

When looking for a cold or flu remedy, consumers should pay special attention to the “active ingredients” label, which lists the medications that work in each product.
In the fall of 2023, an FDA committee met to discuss the status of phenylephrine and, based on new data, unanimously concluded that “current scientific data do not support… the effectiveness of phenylephrine as a nasal decongestant.”
The agency determined that the drug is no more effective than a placebo when taken orally.
However, when phenylephrine is used in medications along with other drugs, which will not be affected, the FDA said it “does not affect the way other active ingredients work to treat the symptoms for which they are intended.”
While the FDA made the recommendation this week, it is only a “proposed order” and will not have an immediate impact on the drugs.
Only a ‘final order’ will affect the products sold, which could have a significant impact on the $1.8 billion in sales generated by these drugs.
The FDA also noted that its conclusion only applies to phenylephrine taken orally, not intranasally in common nasal decongestants.
Phenylephrine became popular in the mid-2000s, when the federal government placed new restrictions on the purchase of pseudoephedrine, an ingredient that can be used to make methamphetamine.
The medication is recommended in doses of 10 milligrams every four hours for temporary relief and works by reducing dilating blood vessels in the nose, relieving nasal and sinus congestion.