Fans of The Simpsons were shocked this weekend when the long-running series killed off a supporting character.
The supporting figure made his debut on the show in December 1989, almost 35 years ago, in the series’ premiere episode, The Simpsons Roasting on an Open Fire, with a Christmas theme.
In the last episode, hilariously titled Cremains Of The Day, viewers learned that Larry the Barfly had died.
Larry had been a member of the drunken group that frequented Moe’s Tavern, and was voiced by Spinal Tap star Harry Shearer, known for portraying a group of characters, including Ned Flanders, Mr. Burns, his assistant. Smithers, Principal Skinner. and Reverend Lovejoy, among many others.
The show, which came under fire from overly sensitive viewers last year for scenes in which Homer comically strangles his son Bart, often featured Larry alongside the much more prominent Barney Gumble, the comic drunk whose muddled pronunciation often steals set scenes. at the bar
Simpsons fans were shocked this weekend when the long-running series killed off a supporting character; still from 2021

The supporting figure made his debut on the show in December 1989, almost 35 years ago, in the series’ premiere episode, The Simpsons Roasting on an Open Fire, with a Christmas theme; in the photo of the ninth season
Although Larry had been around for decades, he never took on a prominent role in any episode and almost nothing was known about the character’s backstory.
That changed with Sunday’s episode, as it was posthumously revealed that his name was Lawrence Dalrymple.
In Cremains Of The Day, Homer and his other drinking buddies explored Larry’s surprising history and realized they barely knew him.
The Simpsons fans reacted on social media with a mix of surprise and mock shock at the news of the minor character’s death.
Not Larry! joked one fan who also posted a gif of Marge and the kids looking stunned.
—I just found out that Larry will be killed tomorrow on The Simpsons. I need a minute,’ another person joked.
Others mocked the late character’s lack of screen presence.
“When Larry isn’t on screen, everyone should be wondering, ‘Where is Larry?'” one fan wrote.
Another added: “I hope we learn something about Larry if we never see him again.”
But others found their life on the margins “really fucking sad and tragic.”
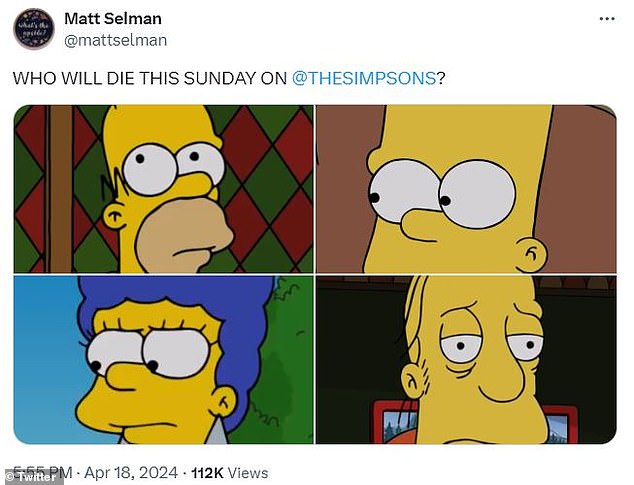
The Simpsons writer Matt Selman, who serves as co-showrunner with Al Jean, poked fun at how his own series was playing with the death of a supporting character.
‘Who will die this Sunday on @TheSimpsons?’ he wrote on X (formerly Twitter) while including images of Homer, Marge, Bart and Larry, which made it clear that the three protagonists were not going anywhere.

In the last episode, amusingly titled Cremains Of The Day, viewers learned that Larry the Barfly (second from left) had died.

In Cremains Of The Day, Homer and his other drinking buddies explored Larry’s surprising history and realized they barely knew him.

The Simpsons fans reacted on social media with a mix of surprise and mock shock at the news of the minor character’s death.
Others mocked the late character’s lack of screen presence.
The Simpsons writer Matt Selman, who serves as co-showrunner with Al Jean, poked fun at how his own series was playing with the death of a supporting character.
One viewer joked about Larry’s cause of death: “Spoiler: autoerotic asphyxiation.”

Even The Simpsons account joined in on the fun by posting a picture of Homer and Larry eating ‘Angel Wings’ at a sports bar in the sky.
One viewer joked about Larry’s cause of death: “Spoiler: autoerotic asphyxiation.”
Even the Simpsons account joined in on the fun by posting a picture of Homer and Larry eating ‘Angel Wings’ at a sports bar in the sky.
Despite the publicity about the death, many fans also admitted that they never knew Larry’s name.
Although The Simpsons has chosen to eliminate some of its less popular characters, it has also brought back iconic faces in recent years, including Jacques, voiced by Albert Brooks, a French bowler who tried to score a strike with Marge.

