An Australian father whose Covid vaccine left him with a paralysing injury that forced him to quit his “dream job” has criticised the government’s treatment of those who suffered adverse reactions to the vaccine.
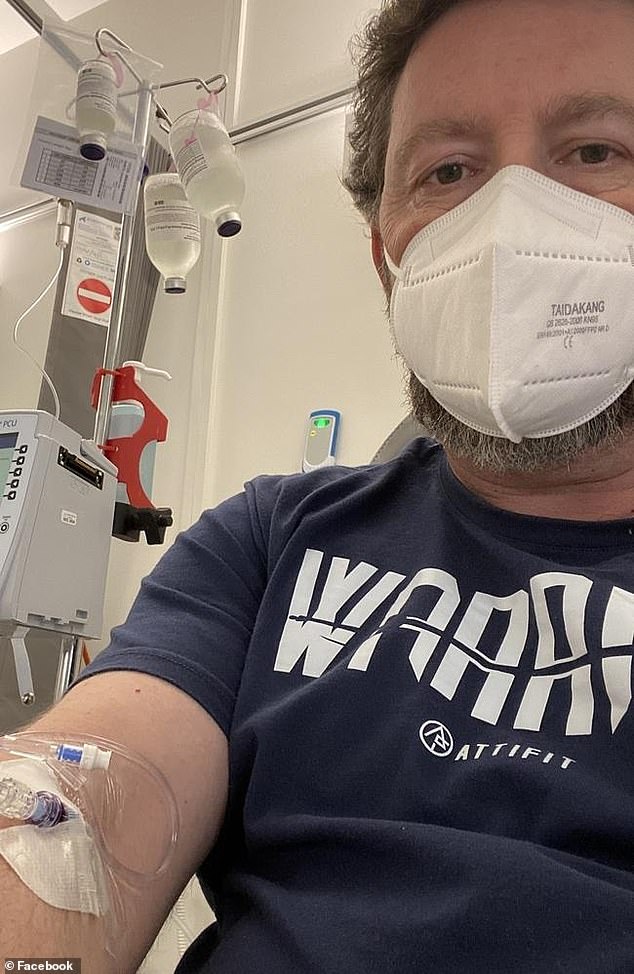
Chris Nemeth, 51, worked in air logistics until he received the AstraZeneca vaccine in July 2021 and developed chronic inflammatory demyelinating polyneuropathy (CIPD) which can cause numbness, pain and even paralysis in the limbs.
Her exhaustive 1,000-page submission to the Covid-19 Vaccine Claims Scheme, including documentation from a GP and a specialist attributing the permanent condition to her Covid vaccine, took more than 455 days to be assessed.
Mr Nemeth said the scheme, which was set up to provide support to those who did their part and got vaccinated but were injured, was a bureaucratic nightmare.
The Melbourne resident, who takes pain medication and also receives an intravenous immunoglobulin infusion in hospital every three weeks, warned there could be “suicides” because the process under the claims scheme is so arduous.
“I had to learn to walk again, I still have constant neuropathy in both hands and feet – tingling, burning, pins and needles, fatigue, my brain doesn’t work like it used to… the neurologist said this is the best I can probably get,” she said. news.com.au this week.
Despite being unable to work since 2021 and having a family to support, he said the claims scheme forced him to jump through many hoops and his application was continually rejected for further information or external legal reviews.
Mr. Nemeth said he was only offered a settlement for his claim this month, but he did not disclose the exact figure.
Chris Nemeth suffered chronic inflammatory demyelinating polyneuropathy (CIPD), a form of Guillain-Barré syndrome, after the AstraZeneca vaccine and requires an intravenous immunoglobulin infusion every three weeks.
The scheme, administered by Services Australia for the Department of Health, has paid out a total of $29.8 million to 378 approved applications, but another 663 are still being assessed.
A government spokesman said the scheme ends on September 30, but all applications received before that date will continue to be processed.
The world’s largest Covid-19 vaccine study, published earlier this year, found there was indeed a link between the AstraZeneca vaccine and some medical conditions, but these were rare and far outweighed by the benefits.
Research from the Murdoch Children’s Research Institute (MCRI) and published in Vaccine, used an electronic database to harness large amounts of data to assess the safety of the Covid vaccine.
The largest study of its kind involved more than 99 million people from Australia, Argentina, Canada, Denmark, Finland, France, New Zealand and Scotland.
The analysis found that vaccine safety signals were warranted for myocarditis (inflammation of the heart muscle) and pericarditis (swelling of the thin sac that covers the heart) after mRNA vaccines.
Guillain-Barré syndrome (where the immune system attacks the nerves) and cerebral venous sinus thrombosis (a type of blood clot in the brain) have been linked to the AstraZeneca vaccine.

Mr Nemeth said the scheme, which was set up to provide support to those who did their part and got vaccinated but were injured, was a bureaucratic nightmare.
The research also found a new sign of acute disseminated encephalomyelitis (inflammation and swelling in the brain and spinal cord) that the researchers said warranted further investigation.
“The findings translate into an extremely small risk and any potential link must be weighed against the well-established protective benefits of Covid vaccination,” said lead researcher Professor Jim Buttery.
Daily Mail Australia has contacted AstraZeneca and the Therapeutic Goods Administration for comment.