Spring Break is off to a tame start in Miami as beachgoers say there are ‘more police than people’ amid a crackdown on out-of-control parties.
Florida officials announced plans to impose some of the toughest restrictions on Miami Beach earlier this month after two deadly shootings erupted last year.
Restrictions include $100 parking fees and early bar closings at 18.00 in an attempt to rein in the chaos.
Young people on TikTok pledged to circumvent the rules, and many have still turned up – albeit in much smaller numbers than in recent years.
The Miami Beach Police Department told DailyMail.com there had been 153 ‘Spring Break-related arrests’ from March 1 to March 10.
The law enforcement crackdown also comes at the expense of local businesses, with owners saying the move is already having a ‘devastating’ impact on their profits.

Spring Break is off to a tame start in Miami as beachgoers say there are ‘more police than people’ amid a crackdown on out-of-control parties


Some revelers have also chosen to enjoy their spring break away from the prying eyes of the police at boat parties and yachts


Countless yachts are out in full force as Spring Break peaks in Miami Beach, Fla., on March 10, 2024


Florida officials announced plans to impose some of the toughest restrictions at Miami Beach earlier this month after two deadly shootings erupted last year
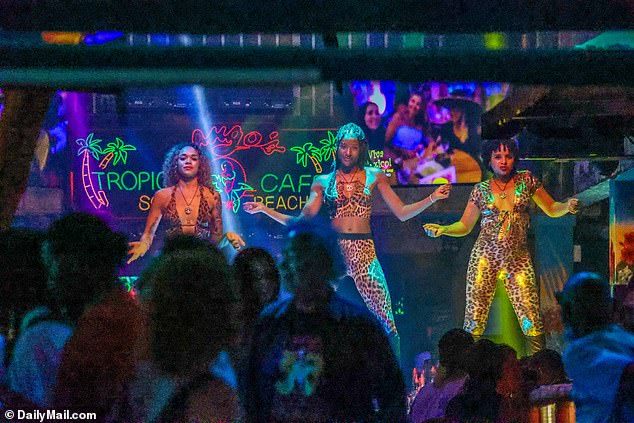
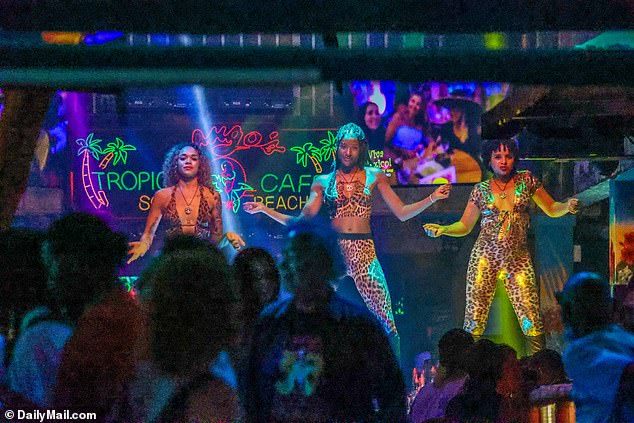
Dancers at Mango’s Tropical Cafe South Beach on Ocean Avenue in Miami Beach, Fla., on March 10, 2024


Restrictions include $100 parking fees and early bar closings at 18.00 in an attempt to rein in the chaos
Photographs from the first day of the long holiday show bikini-clad girls partying on the beach and police officers patrolling the streets in large numbers.
Meanwhile, drone footage of the bigger picture shows how the usual swarms have dropped to more manageable levels this year compared to 2023.
Some revelers have also chosen to enjoy their spring break away from the prying eyes of the police at boat parties and yachts.
‘I’ve seen more police than people,’ said one local Today show on Monday, while another said ‘there are A LOT of cops.’
“We’ve actually been surprised at how calm everything has been,” said another local.
But business owners said the effects had been “devastating” for them, with bars empty for most of the day and restaurants struggling to get orders.
The Miami Beach Police Department made 153 ‘Spring Break-related arrests’ from March 1 to March 10, according to Officer Christopher Bess.
“So far there have been no notable incidents; however, the public can still expect to see a huge law enforcement presence as we move further into the Spring Break season,’ he told DailyMail.com.
Spring Break last year saw 488 arrests in Miami Beach, more than 230 of which were felonies, and more than 100 firearms were seized.
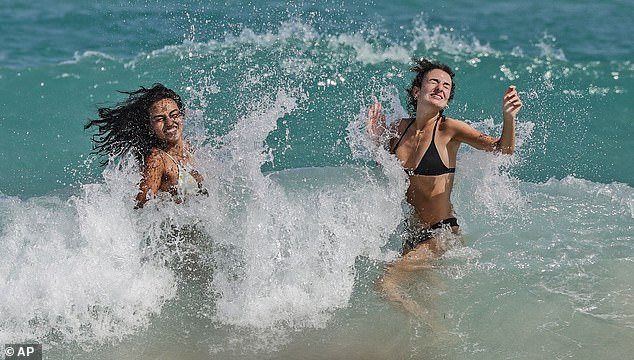
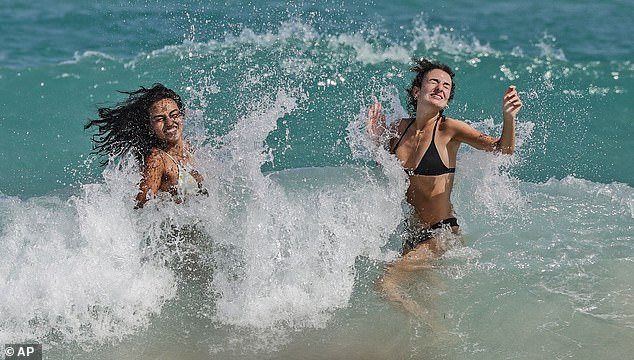
Photographs from the first day of the long holiday show bikini-clad girls partying on the beach and cops patrolling the streets in large numbers


Beachgoers enjoy the beautiful spring break weather in Miami Beach, Fla., Saturday, March 9, 2024


Beachgoers enjoy the beautiful spring break weather in Miami Beach, Fla., Saturday, March 9, 2024


Young people on TikTok vowed to circumvent the rules, and many still showed up in Miami for Spring Break — albeit in much smaller numbers than in recent years
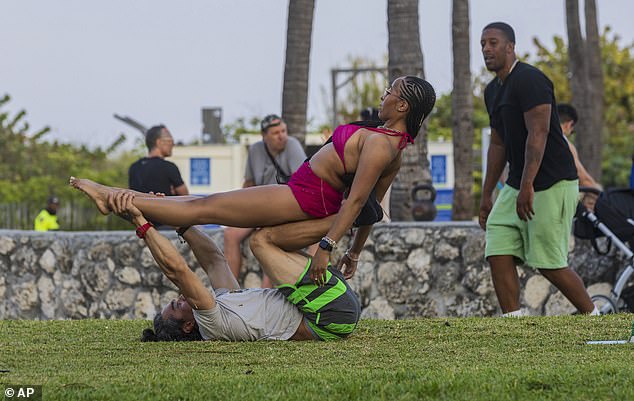
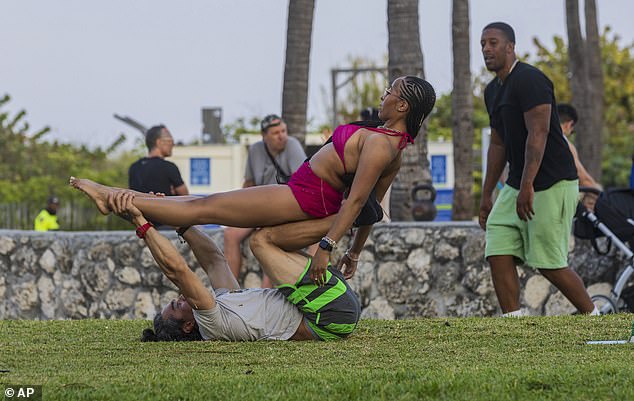
Amia Campbell of Brooklyn does calisthenics with local resident Hivo Gonzalez on Ocean Drive during spring break in Miami Beach, Fla., Saturday, March 9, 2024


Dynamite Dynamite plays his guitar for South Beach visitors during spring break in Miami Beach, Fla., Saturday, March 9, 2024


The usual swarms of Miami Beach Spring Breakers have dropped to a more manageable level this year compared to 2023


Officers work a DUI checkpoint near 5th Street and Meridian Avenue during spring break, Friday, March 8, 2024, in Miami Beach, Fla.


People dance at The Palace on Ocean Avenue in Miami Beach, Fla., on March 10, 2024


Quiet streets and a strong law enforcement presence can be seen at this year’s Spring Break on Ocean Avenue in Miami Beach, Fla., on March 10, 2024


Countless yachts are out in full force as Spring Break peaks in Miami Beach, Fla., on March 10, 2024


Revelers on a yacht off Miami Beach during Spring Break 2024
In a widely circulated video produced by Miami Beach officials, actors discuss the city’s intention to ‘break up with spring break’.
“It’s not us, it’s you,” an actor says to the camera.
“In March, expect things like curfews, bag checks and limited access to the beach,” says another.
A third continues the message, saying: ‘DUI checkpoints, $100 parking and strong police enforcement for drug possession and violence’ are all part of the plan.
Last year, the former Miami Beach mayor said he wanted to cancel Spring Break after fatal chaos broke out on more than one occasion.
A midnight curfew went into effect after two fatal shootings.


People walk up and down Ocean Drive at night on Saturday, March 18, 2023 during Spring Break in Miami Beach
This year, Florida Gov. Ron DeSantis said he and his statewide staff are ‘ready to help our communities maintain order.’
He has ordered 140 state troopers out to various hot spots and added that he is willing to send additional backup if needed.
“We do not welcome chaos,” he said.
‘The state has a lot going on, it’s a fun place to be and we want to see people doing it, but we also want to insist that people respect the law.
“Florida may be popular for Spring Break, but it is inhospitable to criminal activity.”
