<!–
<!–
<!– <!–
<!–
<!–
<!–
A court has found Queensland police and ambulance workers were unlawfully instructed to receive Covid-19 vaccines or face potential disciplinary action.
Covid-19 vaccine mandates for Queensland police and ambulance service workers were made illegally, the state Supreme Court has found.
The court ruled on Tuesday in three claims brought by 86 parties against the Queensland Police Service and the Queensland Ambulance Service over instructions to workers issued in 2021 and 2022.
The rulings did not make a ruling or attempt to make a decision on the transmissibility of a particular variant of Covid-19 or the effectiveness of a particular vaccine.
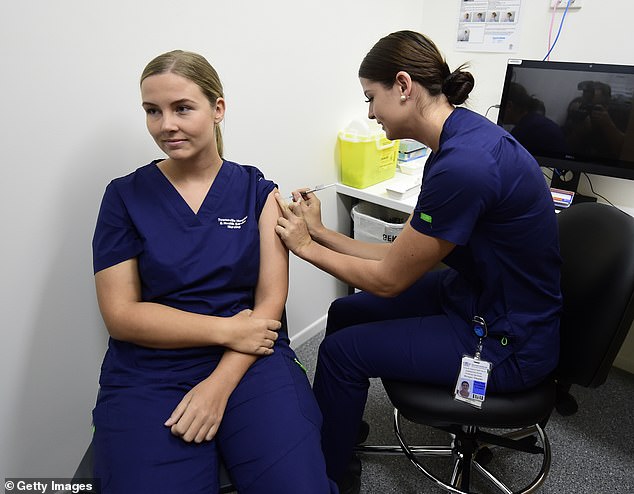
A court found that Queensland police and ambulance workers were unlawfully instructed to receive Covid-19 vaccines (pictured, a nurse is vaccinated in Townsville, Queensland)


Covid-19 vaccine mandates for Queensland police and ambulance service workers were made illegally, the state Supreme Court ruled this week.
Previous instructions required emergency services workers to receive Covid-19 vaccines and booster doses or face possible disciplinary action up to and including dismissal.
The court found that Police Commissioner Katarina Carroll did not adequately take into account human rights relevant to the decision to issue the vaccination mandate.
Former Department of Health director-general Dr. John Wakefield failed to prove that he issued the vaccination mandate under a term implied in employment agreements for ambulance service workers.
As a result, the court found both vaccination mandates “illegal” and of no effect.
The court also found that the instructions limited the workers’ human rights because they had to undergo a medical procedure without full consent, but were reasonable in all the circumstances.
Chief Judge Administrator Glenn Martin said police and ambulance services were trying to prevent their employees from suffering infections, serious illnesses and life-changing health consequences.
“The balance between the importance of the purpose of the limitation and the importance of preserving the human right… is complicated by the fact that these instructions were given in what was, by any measure, an emergency,” he said.
It comes just weeks after a public servant who was forced to get vaccinated against Covid to keep his job won a major legal battle and received compensation.


The court found that Police Commissioner Katarina Carroll (pictured) did not adequately take into account human rights relevant to the decision to issue the vaccination mandate.
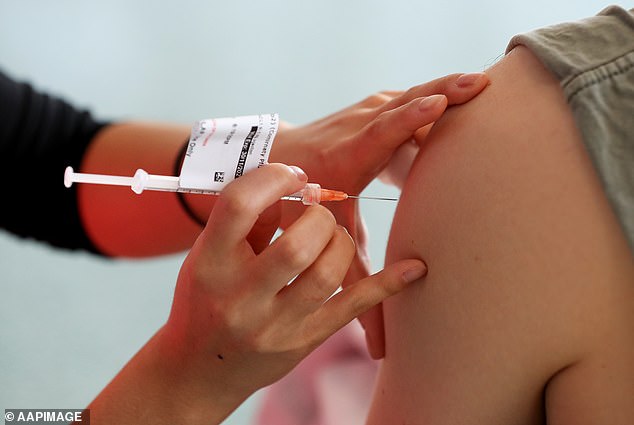

It comes just weeks after a public servant who was forced to get vaccinated against Covid to keep his job won a major legal battle and received compensation for his medical expenses.
Daniel Shepherd, 44, received two Covid-19 vaccines while he was a youth worker at Baptist Care South Australia in 2021 and suffered adverse reactions.
The father of one of them started a new job at the Department of Child Protection (DCP) on October 19 of that year, but on January 28, 2022, he was told he had to receive a booster vaccine to keep his job as a worker. children and youth. .
Shepherd received a Pfizer mRNA injection on February 24, 2022, but a day later he had severe chest pains.
The pain continued to worsen until March 11, when he thought he was having a heart attack and was rushed to Ashford Hospital in Adelaide. There he was diagnosed with post-vaccination pericarditis, an inflammation of the membrane surrounding the heart.
The illness meant Mr Shepherd was only able to work for a few months in a part-time administrative role.
In a landmark ruling, the judge ordered Shepherd receive weekly income support payments and payment for medical expenses.
The ruling came despite SA Health still operating a mandatory Covid vaccination policy for some employees, despite other states abandoning similar policies.
