Nearly nine in 10 patients taking blockbuster weight-loss drugs stop taking them early, a new analysis shows.
Researchers analyzed more than 3,000 U.S. pharmacy claims from patients who were prescribed injections such as Ozempic and Wegovy.
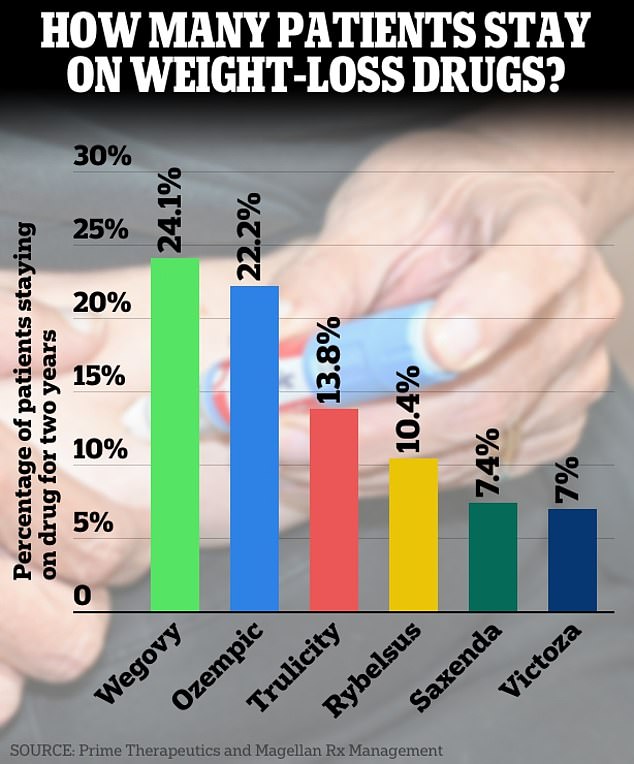
They found that only one in four patients who were taking the drugs for weight loss, rather than diabetes, continued taking Ozempic for at least two years.
And only one in 15 stuck with less popular drugs that work the same way, like Saxenda and Victoza.
The findings come at a time when makers of weight-loss drugs are facing as many as 10,000 lawsuits over serious side effects such as stomach paralysis, tears in patients’ food pipes, blindness and suicidal thoughts.
Only one in four patients taking Ozempic and Wegovy are still taking them after two years, according to a new analysis.

An estimated 15.5 million Americans have taken some form of weight loss medication at some point.
The findings are also significant because previous research shows that when patients stop taking the drugs, up to 80 percent regain the weight they lost.
Researchers cautioned that it is unclear why patients stopped taking the drugs, though they suspect it could be due to side effects such as nausea and stomach paralysis, as well as shortages and lack of insurance availability.
Novo Nordisk, the maker of Ozempic, said it “does not believe these data are sufficient to draw conclusions about overall patient adherence and persistence to various GLP-1 medicines, including our treatments.”
Reports of unpleasant side effects have become more frequent, with patients claiming to have suffered organ failure, suicidal thoughts and having lost the fun of their lives.
The analysis, from pharmacy benefits manager Prime Therapeutics and Magellan Rx Management, reviewed medical and pharmacy claims from 3,364 patients with insurance that covers weight-loss drugs.
This is one of the first long-term studies of the use of drugs that have recently appeared on the market as treatments for diabetes and weight loss.
All patients had received new prescriptions between January and December 2021 and were diagnosed as obese or had a BMI greater than 30.
Participants were prescribed one of the following medications: Ozempic, Wegovy, Trulicity, Rybelsus, Saxenda, or Victoza.
Ozempic, Wegovy and Rybelsus contain the active ingredient semaglutide.
This mimics the hormone GLP-1 (glucagon-like peptide-1), which slows the movement of food through the digestive system, signaling the body that it is full.
Ozempic and Wegovy come in the form of weekly injections, while Rybelsis is available in tablet form.
Liraglutide, which works in a similar way, is the active ingredient in Saxenda and Victoza, while Trulicity uses the GLP-1 agonist dulaglutide.
The mean age of the participants was 46.5 years and 81 percent were women. Patients with type 2 diabetes, for whom the drugs were originally developed, were excluded from the study.
The team found that of all the medications, only about 15 percent of patients were still taking them two years later.
Additionally, 24.1 percent of Wegovy patients continued taking the drugs, along with 22.2 percent of those taking Ozempic.
In the case of Trulicity, 13.8 percent of participants persisted in treatment, along with 10.4 percent of those taking Rybelsus. And only seven percent of patients continued taking Saxenda and Victoza.
According to a 2024 Pew Research report, 8.2 million prescriptions for semaglutide drugs were written in 2021, four times more than in 2019. Now, an estimated 15.5 million Americans have taken a weight-loss drug at some point.


Meredith Hotchkiss (left), 56, told DailyMail.com that her life has been “devastated” by the alleged side effects of the slimming drug Mounjaro. Dina Fioretti (right), 60, from Illinois, said Ozempic made her vomit so much that it made a hole in her oesophagus.
The research team found that one in four patients switched medications at some point during treatment, which they said could be due to shortages or changes in insurance coverage.
They also suggested that patients may have stopped taking the drug because of side effects, as well as shortages and lack of continued insurance coverage.
The findings come at a time when drugmakers such as Ozempic’s Novo Nordisk and Wegovy’s Eli Lilly are facing allegations of several serious side effects.
Meredith Hotchkiss, 56, of Idaho, joined nearly 100 patients in a lawsuit against Eli Lilly and Novo Nordisk after she was diagnosed with gastroparesis.
Ms. Hotchkiss had been taking Mounjaro and Trulicity, another Eli Lilly injection for type 2 diabetes. She was prescribed Mounjaro from July 2022 to about June 2023. She was also briefly prescribed Trulicity from December 2022 to March 2023.
Although she has diabetes, her condition is “well controlled” so she was given off-label medication to lose weight. “I thought if I could lose weight and get Mounjaro then I might as well try it because everyone else is doing it,” she previously told DailyMail.com.
“The doctor told me I could lose weight and that it worked very well. He said I would feel very bad for four weeks and that after four weeks I would feel much better.”
Within a few weeks of starting the medication, her condition worsened and she could not digest anything but cottage cheese, macaroni and cheese and yogurt.
Doctors have also told her she can no longer travel abroad due to her health conditions and she said she now fears she will never be able to eat a solid meal again.
And Dina Fioretti, 60, from Illinois, told DailyMail.com she is suing Novo Norisk after Ozempic allegedly made her vomit so much that it made a hole in her esophagus.