- A map has been divided into 48 counties to show the best player in each area.
- The chart includes some historic stars as well as some darker screams.
- ‘I had a scar on my brain… but I wanted to play’: JAY BOTHROYD’s incredible interview about playing with epilepsy. Listen to the podcast It’s All Beginning
<!–
<!–
<!–
<!–
<!–
<!–
A map detailing the best player born in each English country has served as a timely reminder of the quality of a foreign star.
TO Reddit user has divided a map of England into its 48 counties, including Scotland and Wales, just in case.
In each county there is the name of a player who was born there, considered the best in the area, from Bobby Moore to Steven Gerrard and Jamie Redknapp.
Current stars are on the list, including Real Madrid superstar Jude Bellingham, who was born in Worcestershire and at 20 is already one of England’s best players.
However, one notable name may take some viewers by surprise: it is listed in the county of Yorkshire.

A map has detailed the best player born in each of the 48 English counties, including Jude Bellingham (pictured)


World Cup-winning captain Bobby Moore also appears, although some controversial names are excluded.


Former Liverpool and England captain Steven Gerrard (pictured) is considered the best player born on Merseyside, ahead of Wayne Rooney.
Your browser does not support iframes.
Manchester City striker Erling Haaland was born in Leeds on July 21, 2000 as his father, Alfie, was playing for Leeds at the time of his birth.
Although he is eligible to play for England, he has never wavered his loyalty to Norway, playing for his father’s native country from under-15 level to the senior team.
Several other well-known names appear on the map, such as Bobby Charlton, who was born in Northumberland, Geoff Hurst, who was born in Lancashire, and Martin Keown, who is from Oxfordshire.
Elsewhere, there are more rebellious names including Bristol-born Jack Butland, Kent’s Gary Pallister and Sussex’s Gareth Barry.
Other current players on the map include Eric Dier from Gloucestershire and Dele Alli, who was born in Buckinghamshire.
Another notable named feature from Leicestershire, Hampshire and Northamptonshire is Gary Lineker, Redknapp and Ray Clemence respectively.
With Wales included in his team, Gareth Bale is considered the best player in that country’s history, while Kenny Dalglish takes the title for Scotland.
There were critical responses in the replies, with one fan questioning why Steven Gerrard was listed for Liverpool instead of Wayne Rooney and Peter Crouch instead of Michael Owen for Cheshire.


Meanwhile, Manchester City and Norway striker Erling Haaland was born in Leeds, Yorkshire.
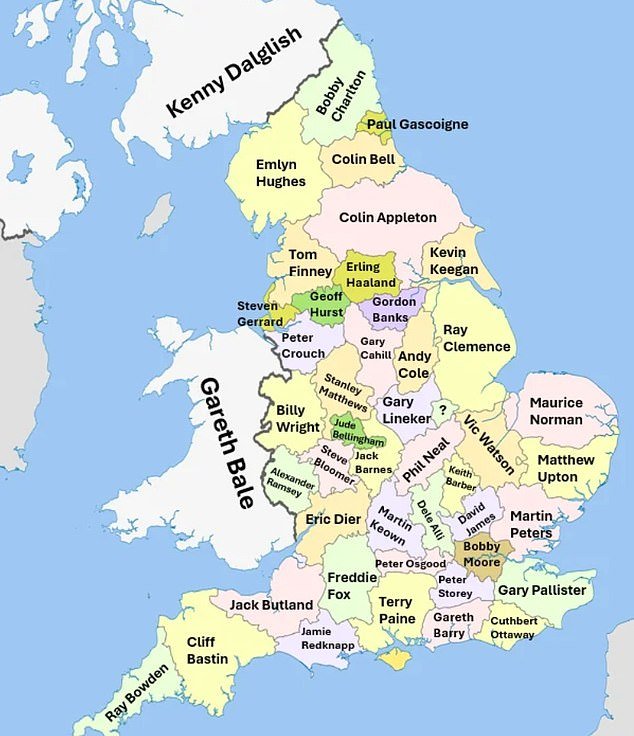

The map details players from each area of England, as well as one from Wales and one from Scotland.
Another user questioned the absence of Harry Kane, while a third criticized the decision to include Paul Gascoigne instead of Alan Shearer for Tyne and Wear.
The only county that did not have a player representing it was Rutland, and the map designer argued that the only player they could find was Harry Springthorpe and that his name was too long to fit on the chart.

