Bandages from some of the most reputable brands, including Band-Aid and Curad, contain dangerous levels of permanent chemicals, a shocking report shows.
Tests by a major watchdog found the chemical fluorine in more than two dozen different bandages that can be found in millions of medicine cabinets across the country.
PFAS chemicals are sometimes used to make adhesives and researchers believe they are products of the normal manufacturing process. Fluorine, which is also used to make rocket fuel, can cause skin burns and eye damage, but is most dangerous when inhaled.
Dr. Linda Birnbaum, a toxicologist and former head of the National Toxicology Program who co-led the laboratory testing, said the fact that dangerous chemicals come into direct contact with open wounds was “concerning.”
PFAS chemicals can easily enter the bloodstream after a person drinks water or eats foods that contain them. Once in the bloodstream, PFAS can lodge in healthy tissues, where they can begin to damage the immune system, liver, kidneys, and other organs.
Four types of Band-Aid brand bandages contained more than 180 parts per million of organic fluoride, a crucial component of the permanent chemicals PFAS.


Thinx Clothes and other vintage products were found to contain anywhere from tens to more than 100 parts per million of the compound.
Environmental health watchdogs had 40 bandages from 18 different brands tested for fluoride, and detectable levels were found in 26 of them.
Mamavation Consumer Watch Blog and Environmental Health News used an EPA-certified laboratory to look for PFAS chemicals in the absorbent pads and adhesive flaps of bandages sold at major retailers, including CVS, Walmart, Rite Aid, Target and Amazon.
Bandages containing high levels of fluoride above 100 parts per million include Band-Aid brands, Care Science, Curad, CVS Health, Equate, First Honey, Rite Aid brand, Solimo (Amazon brand), and Up & Up, the Target brand.
Dr. Birnbaum said, “Because the bandages are placed over open wounds, it is concerning to know that they may also be exposing children and adults to PFAS.
“It is clear from the data that PFAS are not necessary for wound care, so it is important that the industry eliminate their presence to protect the public from PFAS and opt for PFAS-free materials instead.” “.
PFAS substances contain bonds between carbon and fluorine atoms, creating a very resistant chemical that can remain in the environment for years or even decades.
Chemicals are everywhere, most commonly in stain and water repellent products, as well as nonstick cookware.
Teflon, the basic nonstick kitchen coating, is made from a fluorocarbon called polytetrafluoroethylene (PTFE).
PFAS have also been detected in tap water and human blood. A report from the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) found PFAS in the blood of 97 percent of Americans.
According to Mamavation, PFAS in bandages are likely used for their water- and grease-resistant properties.
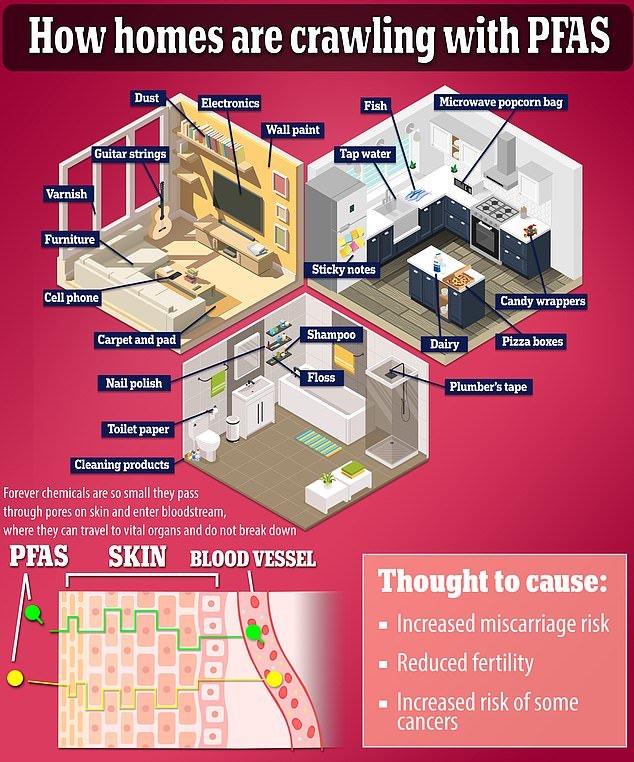

PFAS are a common contaminant in many household items, from kitchen utensils to hamburger wrappers. It can remain in the environment and human tissue for years, even decades, before being eliminated.
Furthermore, PFAS have also been found in popular menstruation products, including those commonly used by teenage girls.
Jessian Choy, who worked at the San Francisco Department of the Environment, decided to test his favorite brand’s vintage Thinx boxers and his BTWN Shorty underwear in the lab.
The test was conducted in 2020 by Dr. Graham Peaslee, who discovered PFAS chemicals in fast food wrappers in 2017, at the University of Notre Dame.
“Organic” products had high levels of PFAS. Thinx had 3,264 parts per million (ppm) and BTWN for teens had 2,053 ppm.
They also had tens to hundreds of parts per million of copper on the inside of the crotch and zinc on both sides.
The investigation generated bad publicity for Thinx, which would later settle a class-action lawsuit for around $5 million. The company refuted the findings and insisted to Dr. Peaslee that its products did not contain PFAS.
Dr. Peaslee said The hill: ‘They called me and said, “Well, this is getting out of hand, can you issue a statement saying it’s safe to use them?” And I said, “I’m sorry, didn’t you hear a word I said yesterday? “I wouldn’t put my damn daughter in this stuff.”
Menstrual underwear is far from the only menstrual products loaded with PFAS. A separate series of Laboratory analysis In 2020 and 2022, Mamavation and Environmental Health News analyzed 46 different pads, panty liners, and incontinence pads for evidence of PFAS.
Fluoride was detected in 22 of them, or 48 percent.
Dr. Birnbaum said, “Dermal exposure to PFAS from menstrual products can be a big problem.” Because vaginal skin is so vascularized, we can anticipate that internal exposure might be a little worse.”
The effects of skin exposure to PFAS chemicals are not well understood, although fluoride exposure can cause serious skin irritation.
Pete Myers, chief scientist of Environmental Health Sciences at Carnegie Mellon University, told Mamavation: “While it is not possible today to answer the question of how much can be absorbed through the skin, we do know that it must be avoid any possible exposure. PFAS should not be found in consumer items, period!’
