A Texas city has been deemed a “cancer cluster” after a government analysis found toxic chemicals in groundwater and soil.
For years, residents of Kashmere Gardens in Houston have expressed concern about an increase in cancer diagnoses. The city of about 10,000 people has seen 150 more cases than expected.
The Environmental Protection Agency (EPA) collected samples from 20 locations around the city and found that most were contaminated with high levels of toxic chemicals, including benzene and dioxin.
The investigation pinpointed the cause to a decommissioned railway facility that burned coal-like tar for nearly 100 years.
Hazardous contaminants seeped into the ground, creating a plume of contaminated groundwater beneath the site, where 110 homes were built.
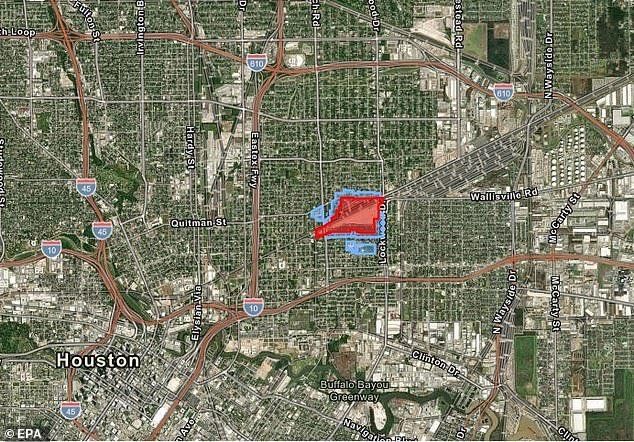
People in the Kashmere Gardens neighborhood of Houston, Texas, have known for years that something was wrong. The EPA finally began testing its soil and found a variety of toxic and carcinogenic chemicals.
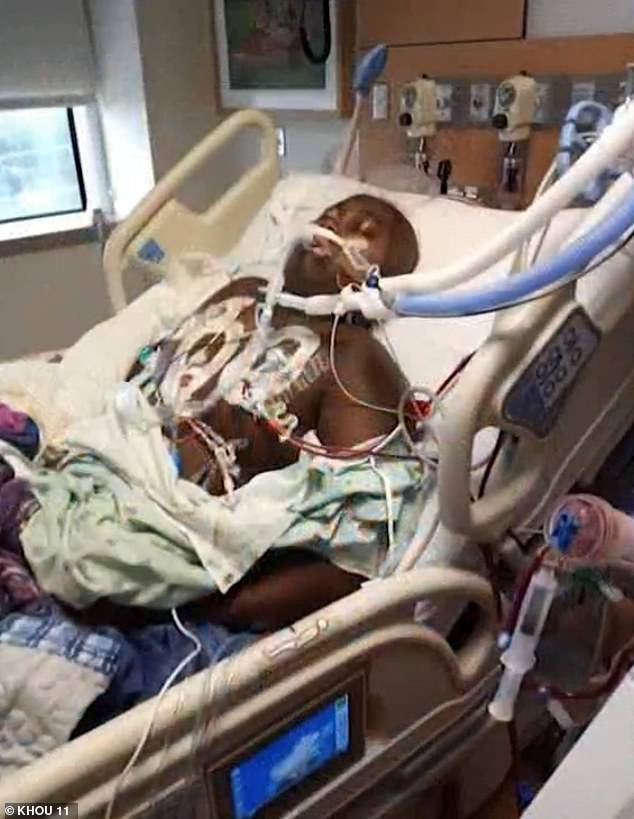

A 2021 study showed that children in the area were being diagnosed with leukemia at a rate five times higher than average. Pictured is Corinthian Giles, 13, whose parents claim she was diagnosed after coming into contact with an old creosote site. She has since died
While the EPA has not finished analyzing all the samples from the area, 41 contained different tar or creosote chemicals.
So far, 51 of their soil and water samples have returned results, but there are There are still 66 samples to be analyzed, – the EPA noted that it could take another four months to complete the investigation.
The agency has set a fall deadline to determine the risk of contamination and whether a cleanup will be necessary.
Sandra Edwards, Fifth Ward resident and founder of IMPACT Fifth Ward Justice, said: ‘Something has been going on here for decades, people don’t drop dead for no reason.
“It just proves that we were right.”
While the EPA analysis shows the community was right, other studies have also shown an increase in cancer throughout the area.
In 2019, the Texas Department of State Health Services found that between 2000 and 2016 there were 15 more cases of esophageal cancer than would normally be expected, and 126 more cases of lung and bronchial cancer than expected.


For years, residents of Kashmere Gardens in Houston have expressed concern about an increase in cancer diagnoses. The city of about 10,000 residents has seen 150 more cases than expected.
Then, a 2021 study showed that children in the area were being diagnosed with leukemia at a rate five times higher than average.
One case was reported in 13-year-old Corinthian Giles, whose parents say he was diagnosed after coming into contact with an old creosote site five years ago.
The boy lost his battle with leukemia and his family sued Union Pacific, which owned the railroad, for wrongful death.
Union Pacific told locals KHO 11: ‘Union Pacific sympathizes with the family and their loss. “We will review the complaint and respond accordingly.”
The railroad said in 2021 that in the past there is no way for carcinogens to enter the human body; EPA analysis found chemicals in groundwater and soil.
Last September, Houston officials approved a $5 million voluntary relocation program in an effort to help Kashmere Gardens residents.
He The former railway station was used to preserve timber from the early 1990s for railway sleepers until 1984.
This process involved burning creosote, a wood treatment that helped prevent the sleepers from rotting.
Creosote can be produced by burning wood or coal tar, but the type that comes from coal tar is most often used to preserve wood.
This chemical is now known to contain many carcinogens, and the type of coal tar is especially loaded with them.
Although the operation was dormant for 40 years, its effects persisted in the form of the so-called “cancer cluster,” the term for an area where cancer is more common than it should be.


The investigation pinpointed the cause to a decommissioned railway facility that burned coal-like tar for nearly 100 years.
A cluster of cancer may be easy to identify, but determining its exact cause may be difficult to prove.
But outspoken local residents, like two-time breast cancer survivor Joetta Stevenson, didn’t let their plight go unanswered.
‘We will not be silent. We will not be silenced,” said Stevenson, president of the Greater Fifth Ward Superneighborhood. ABC13. “I want people to know that this community is no longer the path of least resistance.”
She has no family history of breast cancer, but has had the disease twice.
‘It changed my entire life, my entire existence. “He was very devastating to me as a person, physically, emotionally and financially,” he said.
Stevenson and her neighbors pressured the Houston Health Department to test their soil, and in 2022 they did.
