A Briton has died and dozens more have been sickened in a “superbug” outbreak linked to contaminated eye drops.
Health chiefs blamed the crisis on three different medicinal drops produced in India for patients with dry eyes.
They were all thought to carry an antibiotic-resistant virus that can be fatal for immunocompromised patients.
Bosses at the UK Health Safety Agency (UKHSA) believe the outbreak is now over, with the worst part occurring last autumn when the products were originally recalled.
No details were released about the patient who died.
Brands linked to the outbreak were several batches of AaCarb, Aacomer and Puroptics eye drops.
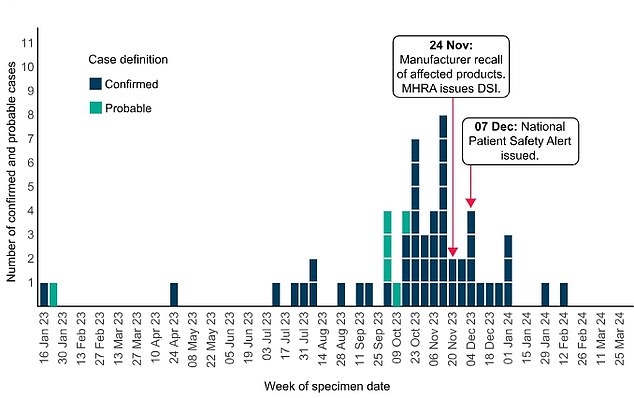

UKHSA said that as of March 21, officials had confirmed 52 confirmed cases linked to eye drop use in Britain, as well as six additional “probable” cases. This graphic shows the timeline of the outbreak in the UK.
Burkholderia cenocepacia, a type of bacteria naturally resistant to antibiotics commonly used to treat these types of infections, was listed as a contributing factor in his death.
The drugs were manufactured by Indiana Ophthalmics, a company based in India.
Several lots of carbomer eye gels from the brands AaCarb, Aacomer and Puroptics were affected.
These products are usually given to patients suffering from dry eyes and can be purchased online for as little as £4.50.
UKHSA said that as of March 21, officials had confirmed 52 confirmed cases linked to eye drop use in Britain.
Six other cases were classified as “probable.”
The youngest patient who fell ill was a baby, while the oldest was 91 years old.
The vast majority were already in the hospital receiving treatment for another problem when staff administered the eye drops without knowing they were using a contaminated product.
Of the cases, 25 were assessed by the UKHSA to have “clinically significant infections” caused by Burkholderia cenocepacia.
Eleven suffered eye infections. Some developed ulcers on the eyeball, while others developed conjunctivitis and a serious “deep tissue infection.”
Nine patients developed respiratory infections and four more suffered blood poisoning.
Most cases were detected between October and December, but one appeared as early as January 2023.
Only after a surge of cases were officials able to link the outbreak to the eye drops.
This led Indiana Ophthalmics to issue a voluntary recall of its three brands of carbomer eye gel in November.
UKHSA also issued a national patient safety alert in December advising all NHS doctors to avoid using carbomer eye gels in high-risk patients, such as those receiving chemotherapy.
In an update yesterday, UKHSA said the outbreak has now slowed and the last case was detected in February.
As such, they withdrew their advice on restricting the use of carbomer eye gel.
But they added that officials will continue to monitor for any new infections.
This is not the first time Indian eye drop makers have been linked to infections.
In April last year there was a wave of eye infections causing death and blindness in the US linked to products manufactured by Global Pharma Healthcare in India.


The medication involved in the outbreak was manufactured by Indiana Ophthalmics, an eye medication company based in India.
U.S. inspectors who visited the company’s plant in Tamil Nadu state found dirty equipment and clothing, missing safeguards and procedures and dozens of other problems.
In a document the U.S. Food and Drug Administration wrote, they found poor cleaning throughout the factory, as well as significant gaps in written procedures and employee training.
Surfaces that touched the containers “were not cleaned, disinfected, decontaminated or sterilized” and there were gaps or discrepancies in records related to how machines and key areas were cleaned.
The surfaces were not easy to clean, as one room had “soft, unsmooth and cracked caulking, protruding nails and nail holes” in the walls.
The company also did not record or have rules about how many times sterilized coveralls could be reused in the company’s clean room after washing, where the eye drops are made.
An inspector also saw a “black, brown, greasy deposit” on a part of one of the company’s machines used to bottle its product.
Global Pharma Healthcare Pvt Ltd was found to have skipped important tests to prove its products were sterile.
It also did not verify the ingredients it used to make the products, based solely on what its supplier said.
