More than 600,000 bottles of a blood pressure medication sold in the United States have been recalled over fears they may be contaminated.
Ramipril is prescribed to more than 2.4 million Americans each year and is used to lower blood pressure by dilating blood vessels.
But the FDA issued a recall because the drugs were made with ingredients from a supplier in India whose factory had not been inspected or approved by the agency.
This raises concerns that the capsules may be at risk of contamination.
But the overall risk to the public is low, the agency said, and no adverse events have been reported to date from taking these medications.
The recall affects capsules of the drug manufactured by the Indian company Lupine Pharmaceuticals and sold in concentrations of 2.5 milligrams (mg), 5 mg and 10 mg.
They are in bottles of 90, 100 or 500 capsules and their expiration date is until July 2026.
The capsules were manufactured in Goa, India, and the recall was launched on October 23 for the 10 mg and 5 mg strengths. This was expanded to include 2.5 mg doses on November 19.
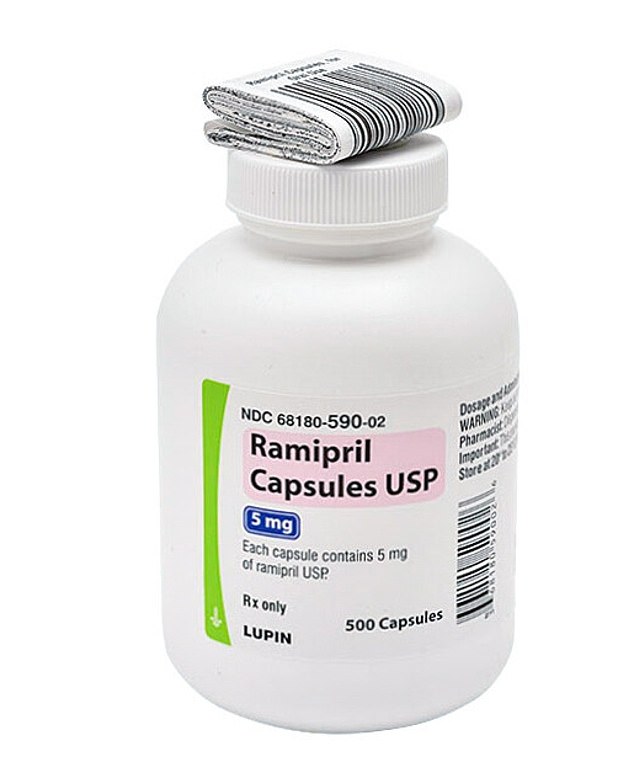
Pictured above is a bottle of ramipril with a 5 mg strength containing 500 capsules and was sold by Lupine Pharmaceuticals. Versions of this medication are being recalled.
An FDA inspection revealed that an active pharmaceutical ingredient (API), the main ingredient in a drug that causes its effect, was made by a separate manufacturer not approved by the agency.
The recall is classified as Class II, which is issued when a product has a low chance of causing serious injury or death.
A Class I action is when there is a reasonable probability of injury.
A total of 350,000 bottles of 10 mg capsules, as well as 146,000 bottles of 5 mg capsules and 110,000 bottles of 2.5 mg capsules, have been recalled.
The bottles were sold at at least 30 vendors across the country, whose names have not been identified.
Customers are advised to throw away the bottles or return them to sellers for a full refund.
They are also encouraged to contact their doctors to ask about next steps and new prescriptions.
A complete list of the recalled bottles was revealed on the FDA website.
Lupine Pharmaceuticals did not respond to a request for comment from DailyMail.com.
It comes about two weeks after Indian laboratory Dr. Reddy’s Laboratories recalled 331,500 bottles of medical tablets due to the presence of a carcinogenic material.
Cinacalcet tablets were used to treat hyperparathyroidism, a common disease diagnosed in 100,000 people each year in which hormonal problems cause high levels of calcium in the blood.
But they were pulled from the market after testing by the FDA showed they were contaminated with high levels of an impurity related to nitrosamine, which has been linked to cancer.
The company did not disclose where the tablets were manufactured and it was a Class II recall.
Last year, over-the-counter eye drops made in India were at the center of a recall in several states after they were found to be contaminated with an “eye-melting” bacteria.
More than four people died of sepsis after using the eye drops, 14 people experienced vision loss, and more than 80 were infected.
The eye drops were sold by EzriCare Artificial Tears and as private label products sold at CVS, Walmart, Rite Aid and Target, among others.
The contamination was linked to a manufacturing problem at the Global Pharma Healthcare plant in India, where they were produced. It had inadequate microbial testing and did not include adequate preservatives in the medications.